Masitinib Shows Promise in Study For Treating Progressive MS Patients
Written by |
Progressive multiple sclerosis patients treated with AB Science’s lead compound AB07002 (masitinib) in a Phase 3 clinical trial showed positive results in a non futility test (a test to determine if an experimental therapy shows some sign of efficacy). With the successful completion of the futility analysis, AB Science is justified to continue the remainder of the double-blind, randomized, placebo-controlled study.
“We are very pleased with this outcome as our three on-going Phase 3 studies in neurology, in progressive forms of multiple sclerosis, in Alzheimer’s disease, and in amyotrophic lateral sclerosis, are all non futile,” stated Alain Moussy, CEO and co-founder of AB Science, in a news release from the company. “This was not obvious since those three indications have remained so far a challenge for the pharmaceutical industry. This may be indicative of the potential of masitinib for the treatment of neurodegenerative disorders.”
In a futility analysis, a compound is tested in patients to see if it is unable to achieve the defined efficacy objectives of the study. If the compound is declared non futile, then the researchers conducting the trial can continue with the trial because there is reason to believe the compound will achieve its efficacy objectives. A medication to treat forms of progressive multiple sclerosis would be a huge win, as there are no on-label, clinically approved medications for progressive multiple sclerosis patients.
RELATED: Mitochondria May Play a Role in MS Development and Progression
“This positive outcome of the futility test is good news because there is no registered treatment in this indication,” said Professor Ramió-Torrentà, a lead investigator in the trial at Dr. Josep Rueta University Hosptial in Spain. “All drugs registered in relapsing forms of multiple sclerosis, recently or not, have failed so far to demonstrate efficacy in the progressive forms of multiple sclerosis. Furthermore, masitinib is not an immunosuppressive drug, unlike drugs used in relapsing forms of multiple sclerosis.”
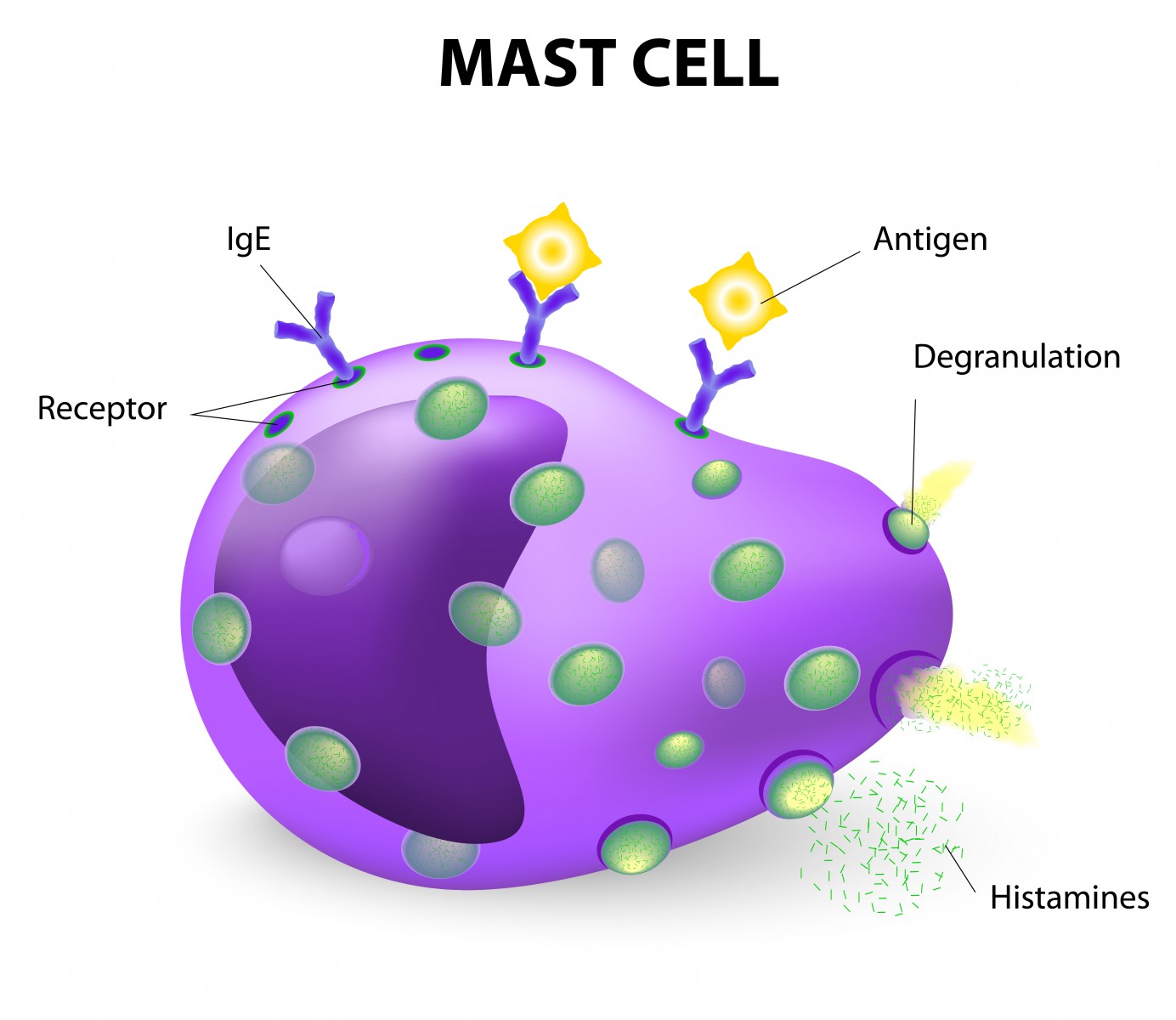
In the 96-week trial, patients with primary progressive or relapse-free secondary progressive multiple sclerosis are being treated with masitinib, a selective tyrosine kinase inhibitor. The compound can control the survival, migration, and degranulation of mast cells by inhibiting key cell signaling pathways. This indirectly controls the amount of proinflammatory and vasoactive mediators release in the nervous system that contribute to disease characteristics.
After one third of patients enrolled for the trial were treated for a total of 48 weeks (halfway through the trial, with an intended full enrollment of 600 patients), they were assessed for changes in the Multiple Sclerosis Functional Composite (MSFC), Multiple Sclerosis Quality of Life 54 Items (MSQOL-54), and changes in the Expanded Disability Status Scale (EDSS). The observed changes were significant enough for masitinib to be declared non futile by the Independent Data Safety Monitoring Committee (IDMC). There were also no major or unexpected adverse events that affected the safety of patients, indicating that the Phase 3 clinical trial is justified to continue forward.


