FAQs about cannabis and MS
Evidence from clinical trials suggests that cannabis products can help to ease some symptoms of multiple sclerosis (MS), particularly pain and spasticity. There is some data that cannabis-based therapies may help manage other symptoms, such as frequent urination and sleep problems, but these effects are less well-studied. For other MS symptoms, like tremor, cannabis therapies are considered unlikely to be beneficial.
Like most other medicinal products, cannabis products may cause adverse reactions; common side effects reported in multiple sclerosis (MS) clinical trials include dry mouth, disorientation, dizziness, and nausea. Cannabis products also can interact with medications, and may pose additional risks to people with certain psychiatric histories or heart conditions. It is generally advised that people with MS should talk with their healthcare team about whether and how it would be safe for them to use cannabis products.
Cannabidiol or CBD is one of the main biologically active compounds in cannabis, though it does not produce a “high” like the other main component, tetrahydrocannabinol (THC). The effects of cannabis products depend largely on the relative amounts of CBD and THC in them. It’s generally recommended that people with multiple sclerosis who are trying cannabis products for the first time should start with a CBD-dominant or balanced product to minimize the likelihood of unwanted effects.
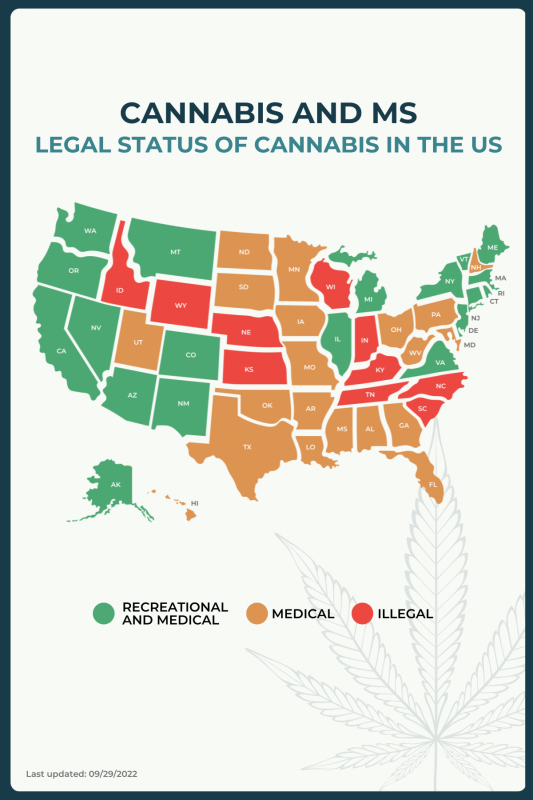
The legality of cannabis use, for recreational or medicinal purposes, varies by country, and within the U.S., cannabis laws differ from state to state. In most states, medical cannabis can be prescribed to ease symptoms of MS, but there are about 10 states in which medical cannabis products are not legal or authorized for use by patients with this neurodegenerative disorder.
Nabiximols is a cannabis-based oral spray that is approved to treat spasticity related to multiple sclerosis (MS) in Canada, Australia, and much of Europe, where it is marketed as Sativex. The therapy, however, is not approved for use in the U.S. Other cannabis products have been evaluated in MS clinical trials, but at present none are widely approved for MS treatment.
Related Articles

 Fact-checked by
Fact-checked by