Remyelination Studies Abound, But How About a Workable Therapy?
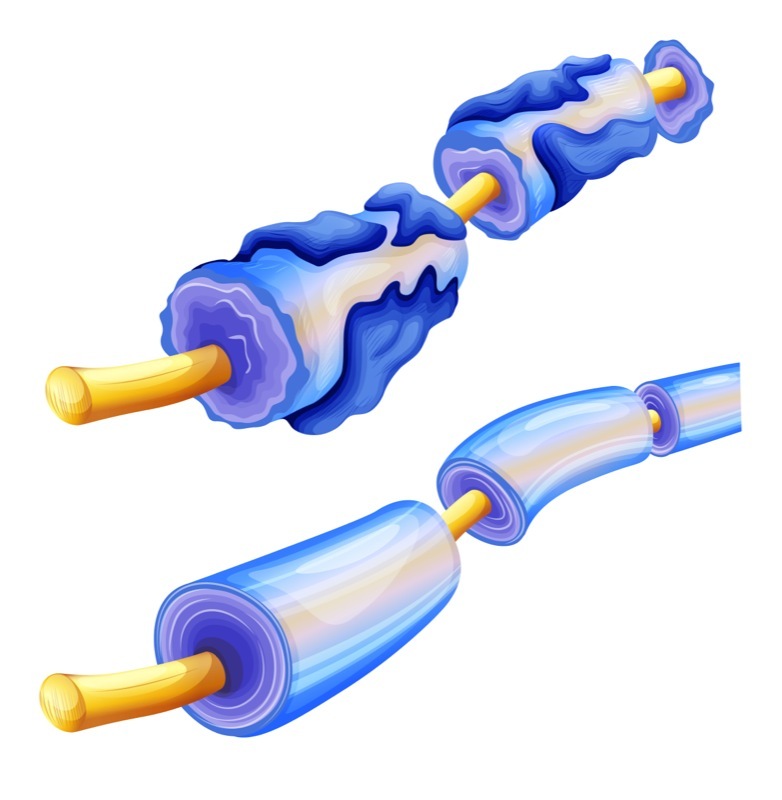

Remyelination at the moment is the buzzword to beat all buzzwords in the world of research into, and treatment for, the vicious disease that is multiple sclerosis.
Now, as you are reading this, you must have some knowledge of MS and are sure to know about the link between the loss of the myelin sheath and MS; that is why I am not going to repeat that here.
But the reason remyelination is making the headlines is that there is so much going on.
Earlier this year, researchers from the University of Cambridge showed that a membrane-bound signaling protein, EphrinB3, blocks the remyelination of damaged neurons in multiple sclerosis (MS). The study, “Antibody-mediated neutralization of myelin-associated EphrinB3 accelerates CNS re-myelination,” uncovered a new target to explore in the search for a new MS treatment.
Next, during a Phase 2 clinical trial testing the efficacy of a common antihistamine, clemastine fumarate, to treat optic nerve damage in people with multiple sclerosis, the drug was able to slightly reverse damage to their visual system.
The study, conducted by researchers at the Multiple Sclerosis Center at the University of California San Francisco, and titled “Positive phase II double-blind randomized placebo-controlled crossover trial of clemastine fumarate for remyelination of chronic optic neuropathy in MS,” was presented on April 19 by Dr. Ari Green, a study author, at the 68th American Academy of Neurology (AAN) Annual Meeting that took place in Vancouver, Canada, through April 21.
Results are exciting, Dr. Green said in a press release, “[I]t is the first to demonstrate possible repair of that protective coating in people with chronic demyelination from MS.”
Third on the list, a substance called fluorosamine was seen to boost remyelination in mice by preventing the synthesis of chondroitin sulfate proteoglycans and by promoting oligodendrocyte function. The findings showed that targeting molecules that block remyelination may be a promising therapeutic approach in multiple sclerosis.
Similarly, fingolimod (Gilenya), a drug approved for patients with relapsing multiple sclerosis to prevent neuroinflammation, may also help these patients by directly enhancing nerve regeneration and increasing myelination in a way that is partly independent of its anti-inflammatory properties.
The study reporting this finding, “Fingolimod promotes peripheral nerve regeneration via modulation of lysophospholipid signaling,” was developed by researchers at the Medical Faculty, Heinrich-Heine-University, in Germany, and published in the Journal of Neuroinflammation.
Most recently, Endece was recently issued an additional U.S. patent for its lead investigational product NDC-1308, being developed to induce remyelination in patients with multiple sclerosis and prevent disease progression.
Now in late preclinical development, NDC-1308 is designed to repair the myelin sheath of demyelinated nerve fibers. The therapy is specifically targeted to heal motor neuron damage, and is intended for use either by itself or in combination with other disease-modifying agents to slow progression.
With the immune system’s attacks on the nerves’ myelin sheaths being such a key part of MS, it is easy to see why remyelination attracts so much interest.
It’s great to see such promising results of research and early stage development, but they are not enough. Let’s get on and find a way to make them work in patients. We need treatments now, not in 10 or more years time.
Note: Multiple Sclerosis News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The opinions expressed in this column are not those of Multiple Sclerosis News Today, or its parent company, Bionews Services, and are intended to spark discussion about issues pertaining to Multiple Sclerosis.




Lauris Freinats
clemastine fumarate ....if there would be same success, hope MS societies around the world will find out that that kind of drug is cheap in a fact, and wouldn't be other MS "miracle" little modified and cost a lot $$$$
Steve
What about MD1003. FDA is sending it back for a third phase 3 trial.
Chris
You can just purchase pure biotin powder and use the dosage in clinical study. I stir it into my morning coffee.
Alishia
Where can I purchase PURE Biotin
Steve Johns
Pure Bulk is the cheapest. It comes in powder form and you can either weigh it out or buy a set of measuring spoons, Pure bulk sell these too, and just use the smidgeon size spoon. I don't think the exact dose is all that important because the dose used is about 10 times that used in supplements and, as with all drugs, is an approximation. People are different sizes and metabolize chemicals at different rates. The most effective dose in the trials was 100milligrams 3 times per day but it was a small trial and a small number of different doses were tried. Have a look at - Biotin for MS Instruction Manual Public Group | Facebook
Steve Johns
The main cause of delays in the availability of these treatments now is our out dated and corrupted regulatory systems. In Australia the Therapeutic Goods Administration (TGA) will only consider whether to approve a drug if the "sponsor" (the company making it) "...applies and supplies supporting documentation." This and 1960's attitudes to drugs that have been used recreationally is why so many symptomatic treatments like Sativex are available in countries such as the USA, Canada, the UK, Spain and New Zealand but not here.
My experience since diagnosis of Primary Progressive Multiple Sclerosis (PPMS) 7 years ago has convinced me that our regulatory system for approval of medical treatments involving drugs is in serious need of reform. It was originally conceived as a protection for the public against snake oil salesmen but the industry was quick to realize that this could be a restriction on profits and they have been very successful in debasing it. It has become a system that can be used by industry and governments to corruptly increase profits.
I have participated in a phase 3 trial of Fingolimod (Gilenya) for 3 years and it has become obvious that this was a strategy to manipulate the system to extend Novartis’s patent for, at least, 7 years. You may be aware that this drug was approved for other types of MS in 2008. Had PPMS been included in the trials at that time it would have been available, or been shown to be ineffective then. Given that they conducted a phase 3 trial, and that this trial was been extended twice for a year each time, it would appear that an effect was demonstrated. By excluding 10% of their ‘market’ they have been able to sell it to 90% of their ‘market’ at $3000/28 days worth for 7 years and will now be able to claim a new application and so start the clock again on their patent for 100% of their market and taxpayers will pay for this.
We have had numerous examples of ridiculous restriction on the availability of drugs to people who need them such as opiates for pain in terminal cancer because they are addictive and cannabis for pain and nausea in terminal lung cancer because smoking can cause lung cancer.
I am supportive of research and trials, I have been involved in 3 to date, as I understand that this, and my illness, is a learning opportunity for all concerned and necessary for the development of treatments. I do understand though that there are aspects of the system that range from out dated and ridiculous to the sort of thing that would normally be associated with organized crime. I think that the MS Advisory Council should be considering strategies to achieve a comprehensive review and reform of the aspects of the drug regulatory system that are shown to be unethical.
Arthur Leeper
Clemastine. My wife was 63 when the trial started at UCSF in San Francisco. The trial was open to those 18-60, or something like that. Fortunately her Neurologist pointed out that Clemastine, in small doses, is available over the counter at your local drug store. Got it? If you want to try it, go down and pick up some Allerghist, or whatever it is called, and take three tablets in the AM and three before bed.
But that's a bit pricy, so you can also do what we did, and go to your local physician (at Kaiser in our case) and get him to give you a prescription. Clemastine was FDA approved in 1980. 36 years ago. Hello? The normal prescription is 2.68 mg, or something like that. To get the 4 mg dose used in the Clinical Trial requires you to take one and a half tablet in the AM, and the same in the PM. The tablets have a nice groove to help you break them.
I suggest starting with a 1/3 dose for a week, see how things feel, then a 2/3 dose for the second week, then the full dose from then on. There are some minor side-effects, but worth it. And there are unexpected benefits, which are an immediate improvement in sleep patterns. Are you waking up three times a night? Clemastine stops that effect.
After twenty months, some undeniable changes can be seen. Some could be seen earlier, as well as the sleep benefits, but improvements are now very, very clear. But it is not a panacea. You can't have MS for more than twenty years and expect to recover overnight. The UCSF research staff is trying to figure out how to eliminate the parts of the compound that cause side-effects (dizziness, etc) so they can bump up the positive parts ten times higher. That's a wonderful idea. I think the world of UCSF for the work they're doing. And you can become part of a rogue clinical study, by joining in, by working with your Doctor or Neurologist. Since Clemastine has been around since 1980, it has a well-documented history. You may not react well to it, or you may. All you can do is try for a couple of months and see if you can tolerate it well. The better sleep part is a huge benefit, all in itself.
But Gilenya? The makers of it recently said "Ooopsy, Gilenya can cause Shingles". As I can testify. Shingles and MS are a toxic combination. Before you even THINK of taking Gilenya, get a Shingles Vaccine, no matter what your age is, okay? My wife got Shingles for two years, and it's far from a trivial condition, for those who already have MS.
Amy
There was also a study with mice that showed possible improvement in their myelin after fasting for 3 days. https://news.usc.edu/101187/diet-that-mimics-fasting-may-also-reduce-multiple-sclerosis-symptoms/
You can find the clemastine fumarate over the counter at Walgreens.
Carol Giardina
To Arthur Leeper,
What specific benefits other than improved sleep has your wife experienced with Clemastine? Also, are we talking about positive changes in RRMS or in PPMS and SPMS. Thank you.
Arthur Leeper
After 20+ years my wife has SPMS. UCSF was most interested in doing a study on those with RRMS, but I would strongly argue that SPMS is a far better clinical test phase of the illness. Why? Because its normal course is steady and progressive. If there is a change, the odds of it actually just being a "remission" are very low. The most immediate improvement we could see was an improved sleep pattern. By any measure, that is a good thing. There were a host of smaller changes, and VEP tests done at UCSF strongly indicated a lack of decline, and a modest improvement in signal strength in one optic nerve. Again, after twenty plus years, to expect an overnight recovery would be unrealistic, whereas a slow and positive change is encouraging and believable.
I think one has to expect more immediate effects, the shorter the nerve is. The optic nerve is a valuable one to monitor, for that reason. It's very short. Strength in the upper body can be seen, with some lesser good effects on the legs, which have a far longer nerve path. The changes may not be dramatic, but there are several points to consider;
a. no further progression, and
b. some modest improvement
c. a compound that's been in use since 1980, and holds no surprises
The continuing work being done on Clemastine is fascinating. The effort is directed toward figuring out which part or parts of the compound cause "side-effects" (negative reactions), and then figure out a way to pull them out, while leaving the positive myelin-repair aspects of the compound intact, so that the dosage can be increased by something like ten times what is currently being studied and consumed. In theory, that could multiply the speed of remyelination to ten times the current rate. All of that sounds wonderfully obvious and easy, but life only rarely is that simple, but I am very glad they are working on that concept.
Arthur Leeper
Cynthia has SPMS, as does her older brother, so two out of four siblings have somewhat similar lengthy and slow cases, decades, not years. Yes, beyond sleep (which is not a trivial good effect) she seems to have recovery that is trending downward. Her upper body strength is quite good, and getting better from "chair yoga", but I don't think yoga can explain better control of her midriff and legs. When I speak of "control", it should be understood that the effects are only possible to observe over months, not days or weeks. But what is clear, after about 22 months of twice daily dosages of 4 mg, with very few skipped doses along the way, there is a trend which is positive. My guess is that the longer the nerve from the brain, the longer it takes to improve. That probably is a wild over-simplification. There are some modest side-effects, dizziness sometimes. 4 mg is clearly the highest comfortable dose. Which is why UCSF and others are working to take apart Clemastine Fumarate and try to figure out which parts support remyelination, and which trigger side-effects. The goal, I was told, was to find a modified compound that could generate around ten times the positive effect of CF. If they can figure that out, it would be impressive. And remember, Dr. Jonah Chan at UCSF invented a method to permit them to test a thousand compounds in a matter of months, which should permit them to test all the modified versions of CF they are able to create. It all appears quite promising. Stopping MS is important, but just stopping it isn't enough. Complete or almost complete recovery of nerve function strikes me as the key to a normal existence and comfortable lifespan. And that's a good thing!
Maria Edite Ribeiro
About. NDC 1380 ??????
Can you say something
I am takinh Gylenia
Mike
Yes, I also have a couple of questions about NDC 1380. You stated, "The therapy is specifically targeted to heal motor neuron damage..."
Is there a difference between motor neurons and sensory neurons? Why is NDC 1380 targeting only motor neuron damage and not also sensory neuron damage?
Jennifer Kaylin
Jen says: I'm a newcomer to MS. After my third MRI and the detection of a new lesion, my doctors convinced me it was time to take medication. They could see that my comfort zone lay with the most benign medication available, which they said was Tecfidera. I'm now in the low-dose introductory phase, which, after an initial flushing episode, is going smoothly. Any thoughts or advice as I get ready to proceed to the full dosage?