Biogen’s Tecfidera Now The Most Prescribed Oral MS Therapy Globally
Written by |

Cambridge, Massachusetts based Biogen Inc. has reported its second quarter 2015 results, posting a year-over-year seven percent revenue increase to $2.6 Billion in the quarter.
“Biogen remains focused on improving the lives of people living with complex diseases,” says Chief Executive Officer George A. Scangos, Ph.D. “Tecfidera, which is now the most prescribed oral MS therapy globally, is experiencing moderated patient growth following rapid initial uptake. The launch of Plegridy is expanding into new markets, and Tysabri continues to add new patients requiring higher efficacy. Additionally, our hemophilia products are being adopted by an increasing number of patients, and we are working toward the anticipated launches of our first two biosimilar candidates in Europe next year.”
Tecfidera (dimethyl fumarate, formerly known as BG-12) is Biogen’s blockbuster oral therapy for treatment of relapsing forms of Multiple Sclerosis (MS), described by Biogen as an effective, long-term treatment for people living with relapsing forms of MS. Data from two completed clinical trials and one ongoing show that Tecfidera has been proven to reduce rate of MS relapses, slow the progression of disability, and the number of MS brain lesions, while demonstrating a favorable safety and tolerability profile in a broad range of patients with relapsing forms of MS. The drug significantly reduces relapses and disability progression in newly-diagnosed relapsing-remitting MS (RRMS) patients who had highly active disease. Additional data indicate Tecfidera showed strong and sustained efficacy over five years in RRMS patients who were previously treated with an interferon (interferon beta-1a/b [IFN]) or glatiramer acetate (GA). These results were outlined on April 23 in poster presentations at the 67th American Academy of Neurology (AAN) Annual Meeting held April 18–25, 2015 at the Walter E. Washington Convention Center in Washington, DC.
“Taken together, these studies reinforce that Tecfidera delivers robust efficacy when used as a first-line therapy, as well as when it is used after a patient has discontinued interferon or glatiramer acetate treatment,” notes J. Theodore Phillips, M.D., Ph.D. , research investigator at the Baylor Institute for Immunology Research and clinical professor of Neurology at the University of Texas Southwestern Medical Center. “In addition, strong efficacy was observed with Tecfidera treatment in various patient populations including those with highly active MS.”
Tecfidera is taken orally, with or without food. The initial recommended dose of Tecfidera is 120 mg twice a day for a total of 240 mg per day for seven days. The usual dose of Tecfidera after seven days is two 240 mg capsules taken daily, for a total of 480 mg per day.
RELATED: Mitochondria May Play a Role in MS Development and Progression
It is believed that Tecfidera provides a new approach to treating MS by activating the Nrf2 pathway, although its exact mechanism of action is unknown. The drug is thought to inhibit immune cells and molecules, and may have anti-oxidant properties that could be protective against damage to the brain and spinal cord. It is theorized that Tecfidera may work by changing the way the body’s immune system works, to help keep it from further damaging the brain and spinal cord. This pathway provides a way for cells in the body to defend themselves against inflammation and oxidative stress caused by conditions like MS.
DEFINE, CONFIRM, and ENDORSE
The New England Journal of Medicine published results from two phase III clinical trials; DEFINE (Determination of the Efficacy and Safety of Oral Fumarate in Relapsing-Remitting MS) and CONFIRM (Comparator and an Oral Fumarate in Relapsing-Remitting Multiple Sclerosis) for BG-12 capsules in people living with relapsing-remitting MS.
DEFINE
DEFINE (Determination of the Efficacy and safety of oral Fumarate IN relapsing-rEmitting MS) was a global, two-year, randomized, multi-center, double-blind, placebo-controlled, dose-comparison Phase 3 clinical trial that enrolled more than 1,200 patients with RRMS at 198 sites in 28 countries. The study evaluated Tecfidera (240 mg, BID or TID) compared to placebo.involved more than 1,200 participants and was designed to determine if BG-12 (Tecfidera) would be effective in decreasing the proportion of subjects experiencing relapses compared to that of a placebo, while also noting factors of safety and tolerability. The secondary outcomes included progression of disability, frequency of relapses and disease activity as detected by MRI.
The primary objective was to determine if Tecfidera was effective in reducing the proportion of relapsing patients at two years. Secondary endpoints included reduction in the number of new or newly enlarging T2-hyperintense lesions and Gd+ lesions as measured by MRI, reduction in ARR, and reduction of disability progression as measured by EDSS. Safety and tolerability of Tecfidera were also assessed.
Within the CONFIRM trial, the average amount of MS relapses that occurred in one year reduced compared to the placebo. Significant reductions were also observed within the secondary objectives which involved a decrease in disease activity as seen on MRI as well as a fewer number of patients experiencing relapses in the BG-12 group compared to the placebo. Disability progression however, was not reduced significantly in the BG-12 groups compared to placebo.
CONFIRM
CONFIRM (COmparator and aN oral Fumarate In Relapsing-remitting MS) was a global, two-year, randomized, multi-center, placebo-controlled, double-blind, dose-comparison Phase 3 clinical trial reported in the New England Journal of Medicine that enrolled more than 1,400 patients with RRMS at 200 sites in 28 countries. The study investigated Tecfidera (240 mg, BID or TID) compared to placebo and included a reference comparator arm of glatiramer acetate (GA; 20 mg subcutaneous daily injection) versus placebo.
The primary objective was to determine whether Tecfidera was effective in reducing the rate of clinical relapse at two years compared to the placebo group. Secondary endpoints at two years included reduction in: the number of new or newly enlarging T2-hyperintense lesions and the number of new non-enhancing T1-hypointense lesions (MRI scans were obtained at a cohort of sites); the proportion of patients who relapsed; and progression of disability as measured by EDSS. Safety and tolerability of Tecfidera were also assessed.
Post-hoc analysis of two years of integrated data from the DEFINE and CONFIRM clinical studies showed that Tecfidera provided robust efficacy in newly-diagnosed patients who have highly active MS. Patients included in the analysis had been diagnosed with RRMS within one year prior to enrolling in the DEFINE or CONFIRM studies, were either treatment-naïve or previously treated with corticosteroids alone, and met the criteria for highly active MS (two or more relapses within one year prior to enrolling in DEFINE or CONFIRM). Patients included in the analysis were followed for a minimum of five years (two years in DEFINE/CONFIRM plus three or more years in ENDORSE). Compared to placebo controls at two years, the patients receiving Tecfidera showed a significantly reduced annualized relapse rate (ARR). In patients who switched from placebo in DEFINE/CONFIRM to Tecfidera in ENDORSE, improvements were observed in ARR whether they were treatment-naïve or had treated their MS with IFN or GA.
ENDORSE
ENDORSE is an ongoing global, dose-blind, Phase 3 extension study to determine the long-term safety and efficacy of Tecfidera (240 mg, BID or TID). The study has enrolled 1,738 patients with RRMS who completed the DEFINE or CONFIRM studies. Patients who received two years of Tecfidera in DEFINE and CONFIRM continued on the same dose (BID or TID) in ENDORSE. Patients who previously received placebo or GA (CONFIRM only) were randomized 1:1 to Tecfidera BID or TID. Patients participating in ENDORSE will be followed for up to eight years.
The primary objective of the study is to evaluate the long-term safety profile of Tecfidera. Secondary objectives include: long-term efficacy of Tecfidera on clinical outcomes and MS brain lesions on MRI scans and effects of Tecfidera on quality of life measurements. The safety profile of Tecfidera observed in ENDORSE in the presented analysis was consistent with the favorable findings reported in the DEFINE and CONFIRM studies, reflecting a minimum of five years of observation, with no overall increased risk for serious infections observed, including opportunistic infections. The most common adverse events associated with Tecfidera were flushing and gastrointestinal (GI) events. Other side effects included a decrease in mean lymphocyte counts during the first year of treatment, which then plateaued. Tecfidera is contraindicated in patients with a known hypersensitivity to dimethyl fumarate or any of the excipients of Tecfidera.
In summary, these two large-scale phase III clinical trials found that Tecfidera taken twice-daily, could:
• Significantly reduce MS relapses (by 53 per cent).
• Significantly reduce MRI detection of brain lesions (Gd+ lesions by 90 per cent, T2 Lesions by 85 per cent).
• Slow disability progression as measured by the Expanded Disability Status Scale (EDSS) (by 38 per cent).
“Tecfidera continues to demonstrate consistently strong efficacy and favorable safety results that we believe support its position as the new oral standard of care,” observes Gilmore O’Neill, vice president, Multiple Sclerosis Research and Development at Biogen. “More than 135,000 patients have been treated with Tecfidera worldwide since it was introduced to the market two years ago.”
Tecfidera is currently approved in the United States, the European Union, Canada, Australia and Switzerland. Through a robust clinical trial program and commercial launches starting with the United States in March 2013, more than 135,000 patients have been treated with Tecfidera worldwide. To date, the Canadian provinces of Saskatchewan, Ontario and Quebec have added Tecfidera to their drug plan benefits.
A thorough overview of Tecfidera therapy and the clinical trials entitled “Dimethyl fumarate in the treatment of relapsing–remitting multiple sclerosis: an overview” (Therapeutic Advances in Neurological Disorders January 2015 vol. 8 no. 1 20-30doi: 10.1177/1756285614564152) researched and authored by Roberto Bomprezzi Department of Neurology, University of Massachusetts, 55 Lake Avenue North, Worcester, MA 01655, USA is published in the journal Therapeutic Advances in Neurological Disorders.
For additional safety information, and the United States full prescribing information, visit:
https://www.Tecfidera.com
In Biogen’s second quarter 2015 financial results, the company’s multiple sclerosis product sales were $2.2 billion compared to $2.0 billion in the same quarter last year. Tecfidera revenues were $883 million compared to $700 million in the same quarter last year. These results consisted of $721 million in U.S. sales and $163 million in sales outside the U.S. compared to $585 million and $115 million, respectively, in the second quarter of 2014.
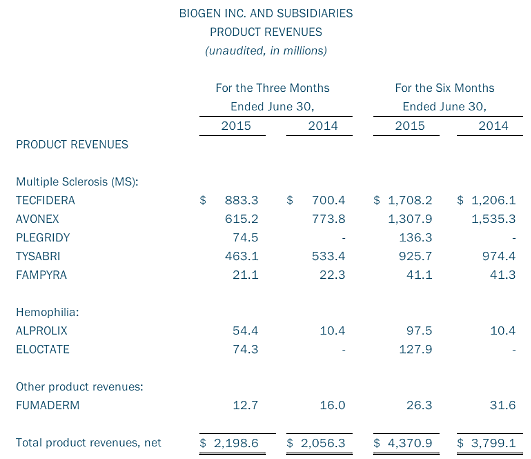
Tecfidera revenues in the second quarter of 2015 increased seven percent from $825 million in the first quarter of 2015. In the U.S., Tecfidera revenues increased 11 percent, which was partially offset by an eight percent decrease outside the U.S. The decrease outside the U.S. was primarily due to lower pricing in Germany, as unit volume increased 14 percent.
In June, Biogen announced that it intends to build a new, next generation manufacturing facility in Solothurn, Switzerland to support its emerging pipeline. Biogen also recently entered into a definitive agreement with Eisai Co., Ltd. to transfer ownership of Eisai’s Research Triangle Park manufacturing campus to Biogen.
In July 2015, Biogen announced that Douglas Williams, Ph.D, Executive Vice President of Research & Development, will leave the Company at the end of July to become chief executive officer of a start-up biotechnology company.
Biogen’s earnings conference call for the second quarter will be available via the Internet, and is accessible through the Investors section of Biogen’s homepage, https://www.biogen.com. Supplemental information in the form of a slide presentation will also be accessible at the same location on the Internet for at least one month.
Sources:
Biogen
Multiple Sclerosis Society of Canada
Image Credits:
Biogen


