Multiple Sclerosis News Today To Provide Live, On-Site Coverage Of 31st Annual #ECTRIMS2015 Congress
Written by |

The 31st meeting of the Congress of the European Committee for Treatment and Research in Multiple Sclerosis (#ECTRIMS2015) is being held this week from October 7 to 10 in Barcelona, Spain. The conference was last held in the Catalonian capital in 1992.
Multiple Sclerosis News Today will be covering the meeting with Dr. Ana de Barros, PhD in attendance as the principal correspondent, backed by the website’s Managing Editor Dr. Patricia Silva, PhD, Isaura Santos, Social Media Director, and a team of staff writers. Our live, comprehensive coverage of the event this year will include live streaming of presentations, exclusive interviews with multiple sclerosis (MS) researchers and influencers in the industry, photos and video from the conference, and daily news coverage of all the stand-out stories from the event.
ECTRIMS notes that The Spanish Neurological Society was originally founded in Barcelona in 1949, and that the strength of Catalan Neurology alongside that of the rest of Spain is still notable today. ECTRIMS is an independent, representative Europe-wide organization devoted to MS, and for a quarter-century has served as Europe’s and the world’s largest professional organization dedicated to the greater understanding of and better treatments for MS. ECTRIMS’ mission is to facilitate communication, create synergies, and promote and enhance research and learning among professionals for the ultimate benefit of people affected by MS.
The 31st Congress will be four days of vibrant and intensive scientific presentations and networking, with many learning opportunities in every current aspect of MS, during which clinical colleagues will impart teaching courses, give plenary talks with updates on the latest available research findings, and present key drug development data through oral and poster communications. A special session devoted to MS nursing is also programmed for this year. ECTRIMS also includes participation from the European Charcot Foundation and industry sponsored symposia. Additionally, for the first time the IMSCOGS (the International MS Cognition Society) will play an official role in ECTRIMS.
 This yearly meeting is one of the most important and powerful concentrations of MS professionals worldwide, and offers those in the field of MS research and drug development an opportunity for bottom-line networking and collaboration opportunities. Multiple Sclerosis News Today‘s coverage this year will be unique to the MS community in that our site-wide coverage will be patient focused, presenting the latest news and developments from the conference in a format that non-researchers and physicians can understand.
This yearly meeting is one of the most important and powerful concentrations of MS professionals worldwide, and offers those in the field of MS research and drug development an opportunity for bottom-line networking and collaboration opportunities. Multiple Sclerosis News Today‘s coverage this year will be unique to the MS community in that our site-wide coverage will be patient focused, presenting the latest news and developments from the conference in a format that non-researchers and physicians can understand.
ECTRIMS works with researchers and clinicians of its member countries and with other organizations that share similar MS-related objectives on a worldwide scale. The organization’s ultimate goals are:
• To support and promote basic and clinical research in MS in Europe and liaise with other related organizations on a worldwide scale;
• To support education and teaching opportunities for professionals that enhance their ability to delivery quality clinical care;
• To act as representative of the MS research community in its member countries;
• To be actively involved in coordinating initiatives in research and clinical care both in Europe and worldwide;
• To organize annual congresses;
• To organize workshops and related teaching activities in the field of MS;
• To initiate, support or endorse European activities of outstanding relevance to MS;
• To encourage young researchers in the field of MS by promoting learning, training and exchange programs.
MS Clinical Trials and Regulatory Affairs
A portion of the conference’s focus this year will address the need to encourage increased MS clinical trial participation as well as best practices for clinical trial design and regulatory issues associated with the disease. Clinical trials are essential to advancing viable therapies for Multiple Sclerosis, and Multiple Sclerosis News Today maintains an MS clinical trial registry to keep patients informed of trials in their area. The International Advisory Committee on Clinical Trials in Multiple Sclerosis is jointly supported by the US National Multiple Sclerosis Society (NMSS) and the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The Committee works to provide perspective and guidance in areas of interest to planning and implementation of clinical trials for new agents for the treatment of multiple sclerosis in international meetings and consensus-building activities, or through subcommittees and task forces on specific topics.
Overall, the 2015 Congress program is designed to tackle topics related to improving future MS disease management. Attendees at the conference will have access to what is in store for diagnosis and differential diagnosis for early MS, what will be the next step in treating patients, and more importantly, how clinicians can shape MS treatment to suit each individual’s profile — maximizing efficacy and safety of complex therapies — and how the environment continues to condition MS evolution.
In support of these objectives, the Congress will map out the epidemiology of MS in less typified geographical areas and address a new emphasis on prevention as well as on research in rehabilitation. Conference goers will also analyze what endpoints are most useful in clinical trials. The program will also devote some time to utilization of new information technologies through the “MS Care 3.0” program to optimize clinical care of patients with MS. In basic research they will review the current role of genetics and new players in neuropathogenesis of MS.
An infographic planner for the Conference sessions abstracts is available here:
https://www.professionalabstracts.com/ectrims2015/iplanner/
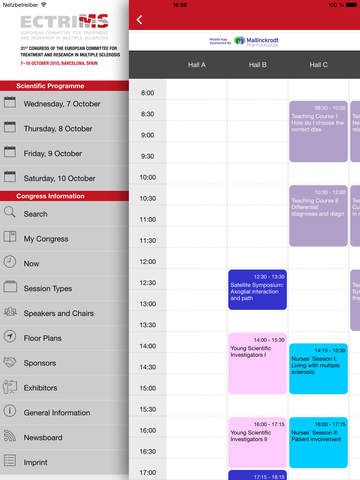 The free ECTRIMS 2015 Congress App for Mobile Devices is available for iOS, Android, and Windows, Download links can be found at:
The free ECTRIMS 2015 Congress App for Mobile Devices is available for iOS, Android, and Windows, Download links can be found at:
https://www.ectrims-congress.eu/2015/congress-app.html
The application will allow participants to navigate through the entire ECTRIMS program from their mobile device, view sessions by day and category, access the abstracts being presented, view posters as a PDF file, locate exhibitors on the map and to create a personalized itinerary.
The application is supported by Mallinckrodt Pharmaceuticals.
The 31st congress of ECTRIMS is accredited by the European Accreditation Council for Continuing Medical Education (EACCME) to provide the CME activity for medical specialists. The EACCME is an institution of the European Union of Medical Specialists, and is designated for a maximum of (or “for up to”) 21 hours of European external CME credits. Each medical specialist should claim only those hours of credit that were actually spent in the educational activity.
The CME accreditation is valid for the main congress program and the teaching courses only and does not cover the company-sponsored satellite symposia.
EACCME credits are recognized by the American Medical Association towards the Physician’s Recognition Award (PRA). To convert EACCME credits to AMA PRA category 1 credits, contact the AMA (https://www.ama-assn.org).
Multiple Sclerosis News Today will broadcast its coverage of ECTRIMS on the website and through our Twitter and Facebook accounts. In addition to using and following the #ECTRIMS hashtag, follow the #msnewstoday hashtag for our exclusive coverage.
Sources:
Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS)


