Dysport (abobotulinumtoxinA) for multiple sclerosis
What is Dysport for MS?
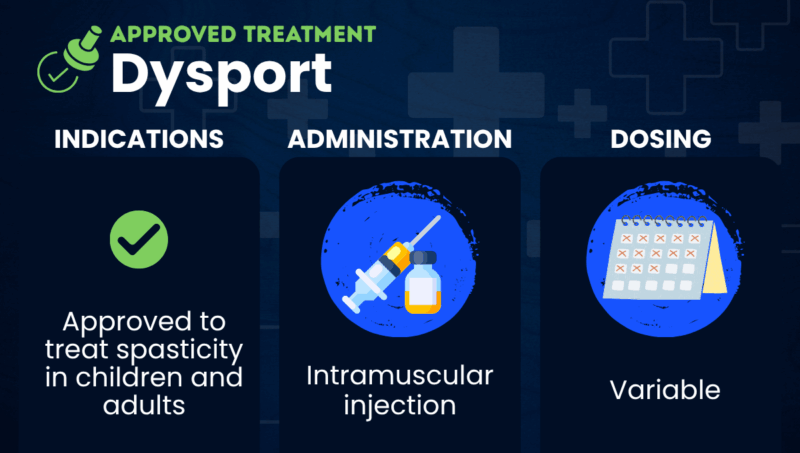
Dysport (abobotulinumtoxinA) is an injectable medication approved to ease spasticity, or muscle stiffness and tightness, one of the most common symptoms of multiple sclerosis (MS).
The medication, marketed by Ipsen, is given directly into the muscle, or via intramuscular injections. It contains a powerful toxin that interferes with the communication between nerves and muscle cells.
Specifically, Dysport prevents the release of the chemical messenger acetylcholine from nerve cells, which would normally bind to receptors on muscle cells and prompt the muscles to contract. By inhibiting these signals, the medication can help ease spasticity, which is caused by overactive muscles.
Although Dysport can ease MS symptoms, it does not alter the overall trajectory of the disease.
The therapy is also indicated in the U.S. for treating cervical dystonia, a condition characterized by spasms in the neck muscles, and glabellar lines, which are the horizontal wrinkles that stretch across the forehead.
Therapy snapshot
| Brand Name | Dysport |
| Chemical name | AbobotulinumtoxinA |
| Usage | Used to ease spasticity |
| Administration | Intramuscular injection |
Who can take Dysport?
Dysport is approved in the U.S. to treat spasticity in adults and children ages 2 and older.
The medication is contraindicated for anyone with:
- a history of hypersensitivity reactions to any botulinum toxin product, cow’s milk protein, or any other component in the medication
- an infection at the proposed injection site
Hypersensitivity reactions are abnormal immune responses, including allergic reactions.
How is Dysport administered?
Dysport is given via intramuscular injections that are administered by a healthcare provider.
As a spasticity treatment, it is given directly into the affected muscles. Dosing is tailored based on the patient’s age, the specific muscles that are being targeted for treatment, and other factors such as the severity of spasticity.
While other botulinum toxin products are available for treating spasticity, the strength of Dysport cannot be compared with other preparations, so the medication is not interchangeable with other botulinum toxin products.
Dysport in clinical trials
The approval of Dysport for spasticity in adults was based on data from two clinical trials, both of which enrolled individuals whose spasticity resulted from stroke or brain trauma. Participants were randomly assigned to receive a single injection of Dysport or a placebo.
- One trial (NCT01313299) enrolled more than 200 adults with upper limb spasticity who received a Dysport or a placebo, administered in the elbow, wrist, shoulder or finger muscles. The results showed that Dysport significantly lowered spasticity in target muscles after one month, with effects lasting up to 20 weeks, or nearly five months, in some patients.
- The other trial (NCT01249404) involved more than 350 adults with lower limb spasticity who received Dysport or a placebo in the gastrocnemius-soleus muscle complex in the calf. A significant reduction in spasticity scores was reported after one month in patients given a higher dose of Dysport. The trial’s long-term extension (NCT01251367), in which participants received up to four additional treatment sessions at least 12 weeks apart, indicated that improvements in spasticity were maintained in the long term.
Dysport’s approval for spasticity in children was similarly backed by data from two Phase 3 clinical studies, which enrolled patients ages 2 to 17 with spasticity due to cerebral palsy.
- A first trial (NCT02106351) enrolled more than 200 children and adolescents with upper limb spasticity, with each given varying doses of Dysport into the wrist or elbow muscles in four treatment cycles. There was no placebo group. Participants given the two higher doses had significantly greater improvements in spasticity at six weeks into every cycle. These effects were maintained about four months after each injection in most patients.
- A second study (NCT01249417) enrolled 235 children with lower limb spasticity who received Dysport or a placebo in the calf muscles. These results showed that Dysport significantly reduced spasticity in the target muscles after one month, leading to improved walking and less episodes of falling and tripping.
Dysport side effects
The most common side effects of Dysport in adults with spasticity are:
- muscle weakness
- falls
- pain in the extremities
The most common side effects in children with spasticity are:
- upper respiratory tract infections
- inflammation of the pharynx, called pharyngitis
- nasopharyngitis, or the common cold
- cough
- fever
The prescribing information for Dysport carries a boxed warning noting that the therapy can spread from the injection area and cause muscle weakness in other parts of the body. This can occur hours to weeks after the injection. It may cause weakness in the muscles needed to breathe and swallow, which can be life-threatening.
The risk of these effects is thought to be highest in children, but symptoms can also occur in adults with preexisting swallowing or breathing issues. People who experience difficulty with speech, swallowing, or breathing after receiving treatment with Dysport are advised to seek immediate medical attention.
Dysport use may also be associated with other safety issues that can warrant additional monitoring. Among them:
- Serious hypersensitivity reactions, including anaphylaxis, can occur. If such a reaction happens, the therapy should be discontinued and appropriate medical care should be given at once.
- There is a potential of passing on infectious diseases, because Dysport contains albumin derived from human blood. However, with modern screening, this risk is extremely low, and no actual cases of disease transmission have ever been documented.
Multiple Sclerosis News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by 

