FDA approves use of robotic exoskeleton for MS rehabilitation
Device allows people with severe mobility issues to stand upright for exercise
Written by |
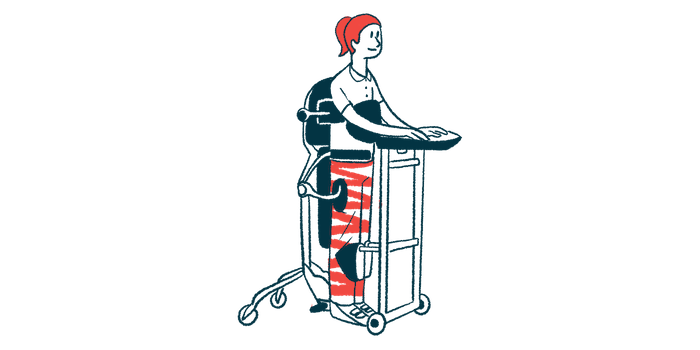
A robotic exoskeleton dubbed Atalante X, which allows people with severe mobility issues to stand upright for rehabilitation exercises, is now cleared by the U.S. Food and Drug Administration (FDA) for use by people with multiple sclerosis (MS) and a broader range of individuals with spinal cord injuries.
Additionally, the FDA approved upgrades to the Atalante X device that developer Wandercraft says will enhance its precision, adaptability, and safety during rehabilitation sessions.
The device had already been cleared in the U.S. for people with spinal cord injuries at levels T5-L5, which generally affect movement in the hips and legs, and for those with paralysis on one side of the body following a stroke.
The new clearance — for individuals with MS and for people with spinal cord injury at levels C4 to L4 — “marks a significant advance in neurorehabilitation and patient access to technology that enables them to walk again,” Matthieu Masselin, Wandercraft’s CEO, said in a company press release. Injuries to the spinal cord at levels C4 to L4 typically result in varying degrees of paralysis from the neck down.
According to Masselin, use of the robotic exoskeleton “enables therapists to personalize training for complex patients, now going as far as to the joint level.” Masselin said this will “help even more people with severely limited upper-body strength to experience upright movement safely and confidently — something which many thought was impossible.”
MS is a chronic neurological condition that affects the brain and spinal cord, causing symptoms such as muscle weakness, spasticity, poor coordination, balance problems, and fatigue. As MS progresses, many people experience increasing difficulty walking or standing, and some may eventually require a wheelchair to get around.
Use of robotic exoskeleton can be personalized by therapists
Atalante X is a self-balancing, hands-free robotic exoskeleton designed to support people with severe walking impairments in standing and walking without relying on crutches or walkers. It can be especially helpful for individuals with limited arm function or cognitive challenges.
By maintaining its own stability, the device enables safe, full-body rehabilitation in an upright position without requiring users to use their hands, allowing therapists to focus on improving not only gait but also posture and balance.
The latest version of Atalante X introduces new features that allow therapists to individually adjust the level of assistance at each joint and fine-tune step length in 5% increments. These features should enable smoother, more personalized adaptation to each patient’s motor abilities, according to the company.
“These capabilities give therapists greater precision to align every session with clinical objectives, comfort, and safety,” the release stated.
The update also adds graphical data in patient reports that now show assistance values below zero to capture resistive movements — instances where the device provides light resistance to help strengthen muscles and refine motor control — giving clinicians a fuller picture of each session.
Other refinements include new training protocols for clinician onboarding and more durable foam supports to improve comfort and device longevity.
People using the device have shown ‘encouraging functional improvements’
The FDA’s decision to expand the device’s approval was supported by data from a retrospective multicenter study involving 547 rehabilitation sessions in individuals with spinal cord injuries in the upper spine — where damage can affect movement in both the arms and legs.
The findings confirmed that the device can be safely used in these patients and provide benefits even to those with tetraplegia, or paralysis in all four limbs and trunk.
“We now have data confirming what we had observed in practice, that Atalante X can be used safety and effectively by people with high-level spinal cord injuries and multiple sclerosis,” said Maria Ida Iacono, global chief regulatory, quality and clinical affairs officer of Wandercraft.
Iacono noted that “participants showed encouraging functional improvements, with a solid safety profile and high levels of user satisfaction.”
Atalante X restores the possibility of upright movement for patients once thought to be too impaired to walk again. We’re building technology that brings humanity back into rehabilitation.
The FDA clearance comes just a few months after the company received expanded CE Mark certification in Europe, clearing the device’s use for a wider range of neurological conditions. These now include tetraplegia and tetraparesis, which is partial loss of movement and strength in all four limbs.
The certification also covered the same upgraded features that have now been cleared by the FDA.
“Atalante X restores the possibility of upright movement for patients once thought to be too impaired to walk again. We’re building technology that brings humanity back into rehabilitation,” Masselin said.