PoNS device for multiple sclerosis
What is the PoNS device for MS?
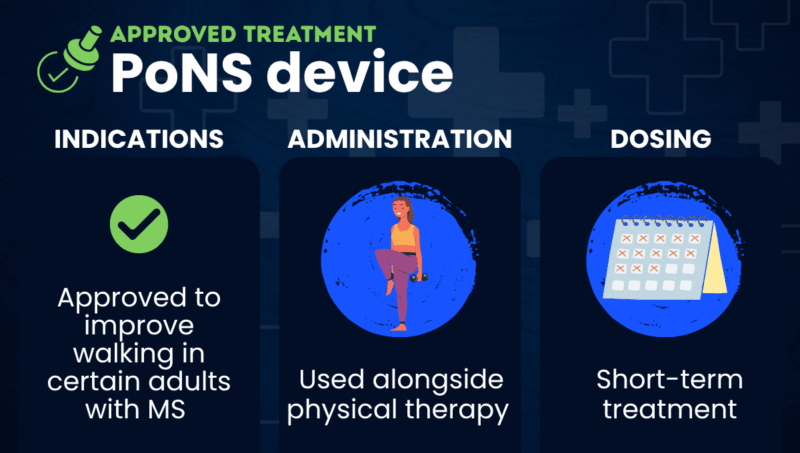
The Portable Neuromodulation Stimulator (PoNS) is a noninvasive medical device approved for use alongside physical therapy to help improve walking in people with multiple sclerosis (MS).
Marketed by Solana, formerly Helius Medical Technologies, the device is used in a short treatment course that typically lasts about 14 weeks.
People with MS often have issues with movement and balance due to problems with the transmission of electrical signals along nerve cells. The PoNS device is intended to improve walking and balance by promoting neuroplasticity, the brain’s ability to rewire in response to new experiences.
The device works by sending mild electrical signals to the brain while patients are doing their exercises. The electrical impulses are delivered to the tongue via a mouthpiece, and then travel along two cranial nerves to stimulate brain regions involved in movement. The mouthpiece is connected, via a cord, to a controller worn around the neck.
When used along with physical therapy, PoNS is expected to help strengthen neuronal circuits related to specific tasks, thereby enhancing the benefits of rehabilitation.
Therapy snapshot
| Brand Name | Portable Neuromodulation Stimulator (PoNS) |
| Usage | Used together with physical rehabilitation to improve walking in MS patients |
| Administration | Used during physical therapy sessions, with stimulation tailored to each patient |
Who can use the PoNS device?
The PoNS device is approved in the U.S. to treat walking deficits due to mild to moderate MS symptoms in adults, 22 years and older. It is used alongside physical therapy, as part of a short-term supervised therapeutic exercise program, and is available by prescription.
Similar approvals exist in Australia and in Canada, where treatment typically lasts 14 weeks and is available for patients ages 18 to 65.
The device is contraindicated, or not recommended, for pregnant women and for people with:
- penetrating brain injuries
- oral health problems, or with recent bleeding, open wounds, or abnormal sensations in the mouth
- chronic infectious diseases
- untreated or poorly controlled high blood pressure or diabetes
- an active or suspected cancer
- sensitivity to copper, gold, or nickel.
It should be used with caution in people who have:
- implanted electronic devices (e.g., pacemakers, stimulation devices, hearing implants)
- metal in their mouth (e.g., piercings, braces, or other orthodontic appliances)
- seizure disorders such as epilepsy.
How is the PoNS device used?
The PoNS device consists of a mouthpiece that is placed on the tongue and a controller that is worn around the neck. It is used during 20-minute sessions of supervised therapeutic exercise, with intensity adjusted individually to each patient.
Exercises, which focus on a person’s walking, balance, and motor control, are guided by rehabilitation therapists who have completed specialized training on the PoNS device.
PoNS device in clinical trials
The approval of the PoNS device for MS was supported by results from two pilot clinical trials that showed its benefits on walking and balance when combined with physical therapy. These benefits have also been confirmed in real-world settings.
- The first clinical trial (NCT04498039), sponsored by the University of Wisconsin, included 20 adults with MS-related walking problems who used PoNS or a sham device during a 14-week intensive exercise program. The program included two weeks of twice-daily sessions in the laboratory, followed by 12 weeks of the same daily routine at home. Results showed patients using the PoNS device had significant improvements in walking after six weeks, which were sustained through week 14.
- The second clinical trial (NCT04496531), sponsored by Helius, involved 14 adults with MS who used PoNS or a sham device while undergoing intensive physical and cognitive training for 14 weeks. Patients using PoNS showed significant improvements in balance after 14 weeks. They also tended to show greater improvements in walking and cognitive function compared with patients using a sham device.
PoNS device side effects
The most common side effects reported with the PoNS device include:
- burning sensation on the tongue
- tingling on the tongue
- sore tongue
- loss of sensation in the tongue
- abnormal food taste.
Multiple Sclerosis News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by 

