FAQs about tolebrutinib in MS
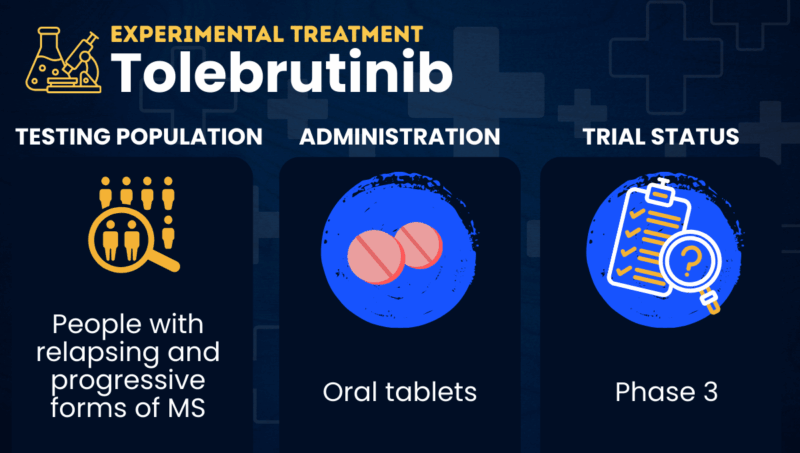
Tolebrutinib is an experimental therapy designed to block the activity of Bruton’s tyrosine kinase (BTK), a protein that is essential to the inflammatory activity of certain immune cells involved in multiple sclerosis (MS). Treatment is expected to reduce the smoldering inflammation that contributes to the progression of disability in MS.
Sanofi has already submitted an application to the U.S. Food and Drug Administration (FDA) requesting the approval of tolebrutinib for treating nonrelapsing secondary progressive multiple sclerosis, and for slowing disability accumulation independent of relapse activity in adults with multiple sclerosis. However, the agency declined to approve the medication in late 2025. It’s not known whether a new application will be filed in the future.
Clinical trials of tolebrutinib have not included pregnant or breastfeeding patients and all trial participants with the capacity to reproduce were required to use effective contraception methods. It is unclear whether tolebrutinib can be safely taken during pregnancy.
Multiple sclerosis can manifest differently in each patient, so individual responses to treatment may vary. In a Phase 2 clinical trial, some early signs of tolebrutinib became evident after about three months, but it’s not known if this was confirmed in larger Phase 3 clinical trials.
Weight gain has not been reported as a side effect of tolebrutinib, but hair loss was observed in some patients who received the medication. Patients who experience unexpected effects of medications should consult their healthcare teams.
 Fact-checked by
Fact-checked by