Sun Pharma-Sponsored Phase 3 Trial to Treat Spasticity Due to Multiple Sclerosis in Recruiting Patients
Written by |
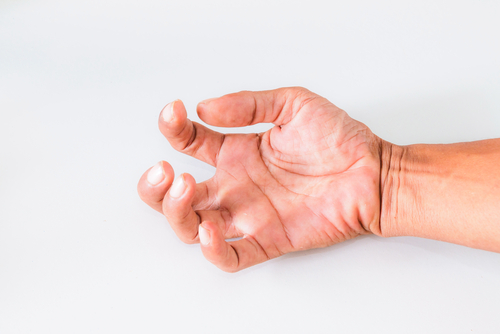
 One of the most common and troublesome symptoms of multiple sclerosis is spasticity. Patients with spasticity experience involuntary muscle spasms and muscle stiffness, which interfere with daily activities. An imbalance in the signals from the brain and spinal cord in the central nervous system increase the excitability of muscles, leading to spasticity. Accordingly, drugs that act on the central nervous system are attractive for treating spasticity.
One of the most common and troublesome symptoms of multiple sclerosis is spasticity. Patients with spasticity experience involuntary muscle spasms and muscle stiffness, which interfere with daily activities. An imbalance in the signals from the brain and spinal cord in the central nervous system increase the excitability of muscles, leading to spasticity. Accordingly, drugs that act on the central nervous system are attractive for treating spasticity.
One such drug is baclofen, a derivative of the neurotransmitter gamma-aminobutyric acid (GABA). Baclofen is used in topical pain creams to relax muscles, is being studied to treat alcoholism, and has shown efficacy against hiccups. A phase 3 clinical trial sponsored by Sun Pharma Advanced Research Company Limited is testing the safety and efficacy of Baclofen ER capsules in patients with spasticity due to multiple sclerosis. The trial is recruiting at 25 locations throughout the United States, and one recruiting location is Future Search Trials of Neurology, LP in Austin, Texas. It is estimated that 300 men and women, 18 years of age and older, will be recruited for the study, and 214 were still needed as of April 1st. One inclusion criterion is that the patient has been taking a stable daily dose of Baclofen IR (immediate release)
Baclofen ER (extended release) is a drug version might be effective even when taken less frequently. The trial is set up to administer immediate release capsules to patients daily for one week before administering Baclofen ER capsules in an open-label fashion daily for up to 16 weeks. After this period, the trial will transition to a double-blind intervention, where patients will receive either Baclofen ER or placebo for four weeks.
[adrotate group=”4″]
Patients will be evaluated for treatment failure during the double-blind period and adverse effects throughout the trial. As a secondary evaluation, patients will be given the Subject Global Impression of Severity (SGIS) assessment. Interested patients are encouraged to look at the recruiting locations and make contacts as appropriate.

