A Step Toward Multiple Sclerosis Treatment? Phase 2 ANTI-LINGO-1 Results Announced
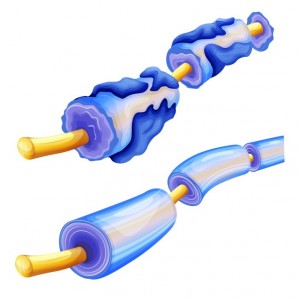 Biogen Idec, a Cambridge Massachusetts Biotechnology company, has released results from its Phase 2 acute optic neuritis (AON) RENEW trial. The trial tested anti-LINGO-1, a medication that restores myelin — a fatty substance that facilitates nerve cell impulses by wrapping around them and providing insulation. The trial results indicate that anti-LINGO-1 could help to repair the damaged visual system.
Biogen Idec, a Cambridge Massachusetts Biotechnology company, has released results from its Phase 2 acute optic neuritis (AON) RENEW trial. The trial tested anti-LINGO-1, a medication that restores myelin — a fatty substance that facilitates nerve cell impulses by wrapping around them and providing insulation. The trial results indicate that anti-LINGO-1 could help to repair the damaged visual system.
RENEW is one component of Biogen Idec’s program to develop anti-LINGO-1, which includes the SYNERGY multiple sclerosis trial. SYNERGY is an ongoing, separate Phase 2 study of anti-LINGO-1 in people with relapsing multiple sclerosis. Biogen Idec expects results for the SYNERGY trial in 2016.
In multiple sclerosis, the deterioration of myelin due to an autoimmune response accounts for loss of movement and other debilitating neurological symptoms. Targeting LINGO-1 might be a way to repair and increase myelin, as well as to help other nervous system cells to survive. LINGO-1 stands for: leucine-rich repeat and Ig domain-containing, Nogo receptor interacting protein. This protein is found in the central nervous system on two types of cells: oligodendrocytes; which provide myelin, and neurons; the information-processing cells of the nervous system. The normal function of LINGO-1 is to stop neurons and oligodendrocytes from further developing once the nervous system is fully formed. Inhibiting LINGO-1 could enable repair following injury.
AON is a disease of the visual system, in which loss of myelin sheath and neuron injury occur, causing damage to the optic nerve. Loss of vision can result. Because myelin loss in AON is well-understood and in one area of the body, studying anti-LINGO-1 in this disease is considered a good first step to test if the drug works. The next step will be to study multiple sclerosis, which is a more complex disease.
Results of the RENEW trial showed that giving patients anti-LINGO-1 improved standard measurements of vision and eye function by 24 weeks following the start of medication, compared to a group that received a placebo. Eighty-two individuals were included in this study.
“We believe the RENEW results are encouraging, as this is the first clinical trial to provide evidence of biological repair in the central nervous system (CNS) by facilitating remyelination following an acute inflammatory injury,” remarked Alfred Sandrock, M.D., Ph.D., group senior vice president and chief medical officer at Biogen Idec. “We look forward to the SYNERGY results in 2016 to further advance our understanding of this molecule in MS, including a full dose response. The totality of the data from the two Phase 2 studies may provide us with a clearer understanding of anti-LINGO-1’s clinical potential.”
Further data from the SYNERGY trial will shed light on whether anti-LINGO-1 can restore myelin and reduce symptoms in multiple sclerosis, providing more progress toward a possible treatment.






