Acorda Announces Phase 1 Results for Remyelinating Antibody in Multiple Sclerosis
Acorda Therapeutics, Inc. has released results from a Phase 1 clinical trial of rHIgM22 for multiple sclerosis, showing that the medication is safe and produces few side effects.
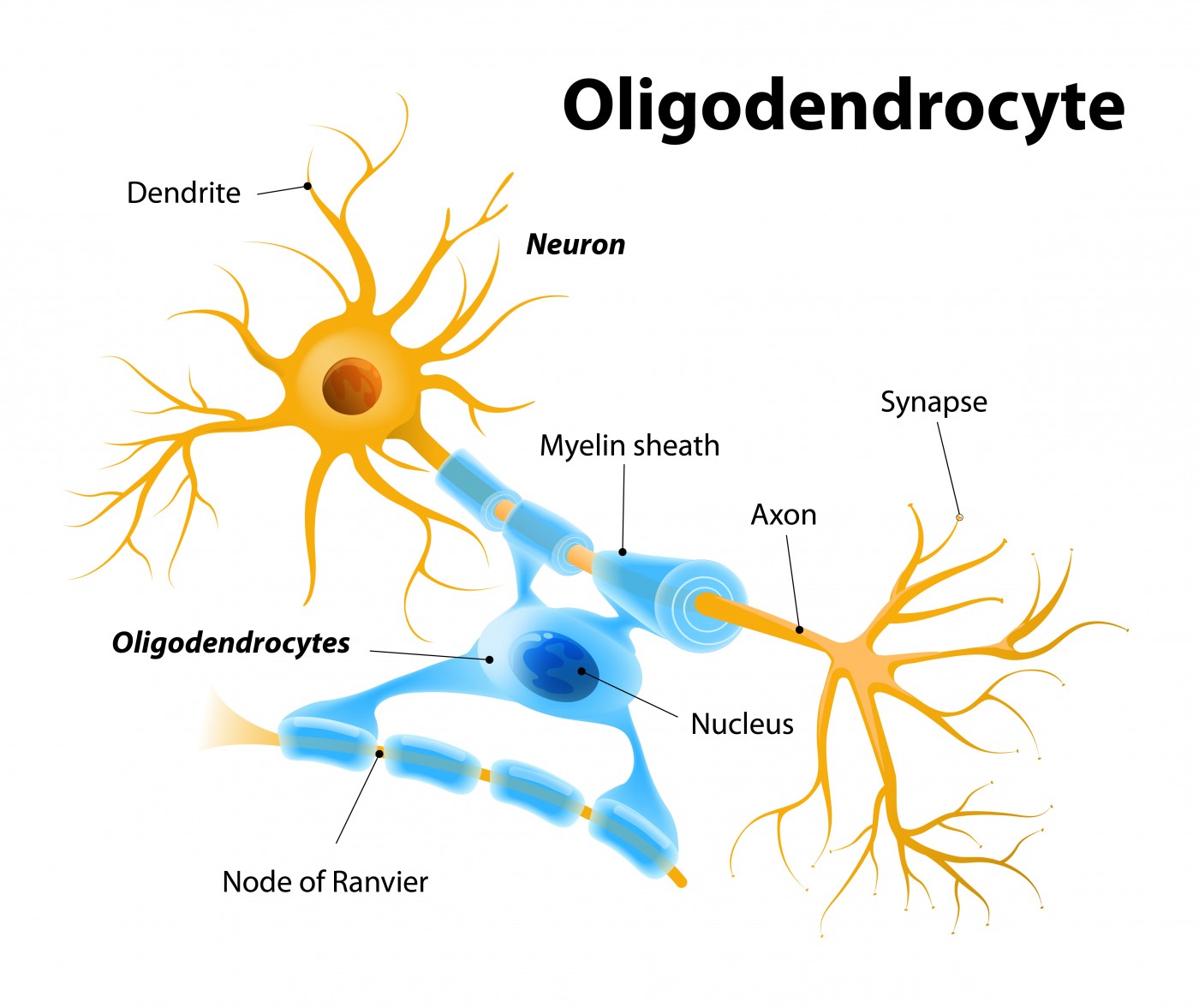 rHIgM22 is a medication that may induce the re-wrapping of the myelin sheath that surround nerve cells, allowing them to conduct impulses from one to the other. The symptoms of multiple sclerosis are believed to be due to an immune attack on the body’s own myelin, which can cause unpredictable loss of movement, sensation, vision problems and feelings of pain.
rHIgM22 is a medication that may induce the re-wrapping of the myelin sheath that surround nerve cells, allowing them to conduct impulses from one to the other. The symptoms of multiple sclerosis are believed to be due to an immune attack on the body’s own myelin, which can cause unpredictable loss of movement, sensation, vision problems and feelings of pain.
The medication rHIgM22 is a recombinant human monoclonal antibody that was originally identified in the laboratory of Moses Rodriguez, M.D. at the Mayo Clinic. In animals, rHIgM22 protected oligodendrocytes, the cells that produce myelin, from death. It also caused oligodendrocytes to repair regions of the nervous system that had lost myelin. With rHIgM22 treatment, animals with experimental MS also regained some motor function.
The Phase 1 study lasted up to six months. In the first phase, fifty participants with any type of MS received a single dose of rHIgM22, with five dose different dose levels studied by the researchers. Eight people were given each dose, and two per dose group were given placebo. None of the doses caused excessive side effects compared to the placebo, or problems that would limit the use of this medication. During the study, people with MS continued on any medication that they were taking for the condition.
[adrotate group=”4″]
In the second study phase, 21 people with MS who had never received any medication for the condition received either placebo or one of the two highest doses of rHIgM22. The study subjects were examined for six months. Again the medication was found by the investigators to be safe and tolerable. In the second part of the study clinical, imaging and biomarker measurements were also taken. This data is still being analyzed and will be released later.
Overall, the most common side effects were: headache, contact dermatitis, MS relapse, infusion site hematoma, fatigue, arthralgia, back pain, muscular weakness, neck pain, pain in an extremity, pruritus, contusion, and flushing. No participants dropped out of the study due to these side effects.
Acorda will continue to develop the medication based on these results. Anthony Caggiano, M.D., Ph.D., Acorda’s Senior Vice President of Research and Development stated “We’re encouraged by the outcome of this trial, which showed that rHIgM22 was well-tolerated at all of the dose levels we studied. We are currently developing the protocol for our next Phase 1 clinical trial of rHIgM22. The data from this study will help inform the design of the next trial, which will enroll people with MS who are experiencing an active relapse.”






