Link Between MS Therapy Tysabri and Melanoma Possible, an Adverse Reactions Watchdog Group Says
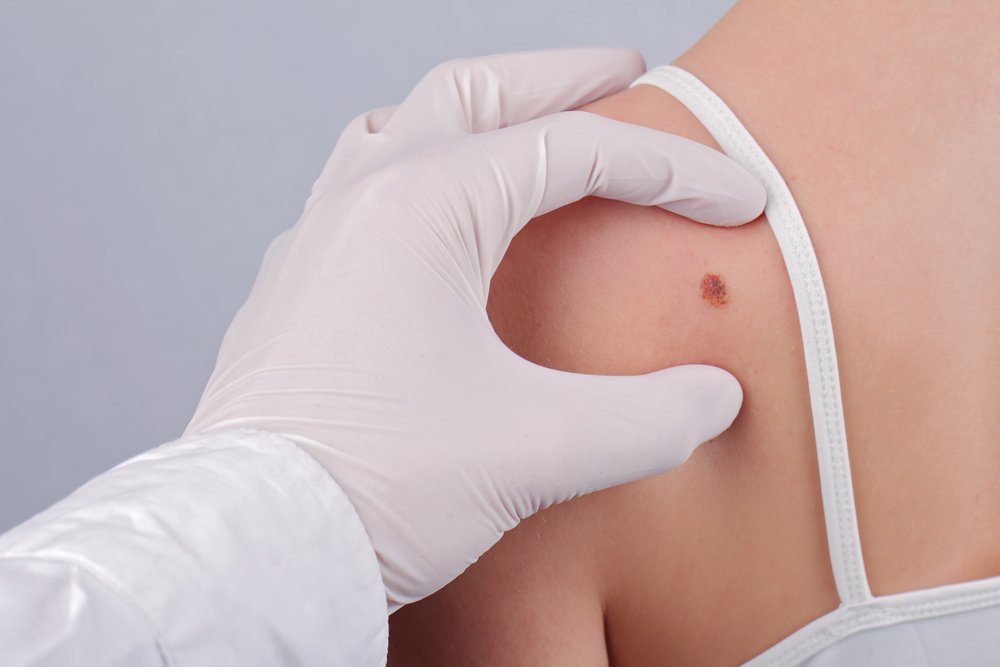
The multiple sclerosis therapy Tysabri (natalizumab) could trigger melanoma, the Southern Network on Adverse Reactions (SONAR) has warned.
Although its investigation failed to demonstrate that melanoma is more common among Tysabri-treated MS patients than in the general population, unusual features among the patients raise concerns about a possible link, the organization said.
Contending that current monitoring efforts are inadequate, it suggested improvements that could generate a better understanding of the relationship between Tysabri treatment and cancer.
The organization’s report, published in the journal Cancer Medicine, was titled “Melanoma complicating treatment with natalizumab for multiple sclerosis: A report from the Southern Network on Adverse Reactions (SONAR).”
SONAR is an organization that was formed in the Southern United States in 2010 to investigate adverse drug reactions that regulators might not be aware of. Its goal is to reduce the time it takes between detecting an adverse reaction and have regulators act on it.
A case that a SONAR investigator came across led to the group investigating possible links between Tysabri and melanoma. A 43-year-old woman developed melanoma in her urethra, the tubing that drains urine from the bladder, after being treated with Tysabri for about two years.
Melanoma is most often a skin cancer that is related to sun exposure, but the woman had no skin lesions. After extensive surgery, she relapsed and died when the cancer spread to other parts of her body. She had declined anti-cancer treatment.
The case prompt SONAR to look for similar cases. Its investigators found seven studies that involved Tysabri-treated MS patients developing melanoma.
In addition, they looked through the U.S. Food and Drug Administration’s Adverse Event Reporting System (FAERS) and the Tysabri Safety Surveillance Program. The surveillance program is part of the Tysabri Outcomes Unified Commitment to Health (TOUCH) database run by Tysabri’s developer, Biogen,
The research team found 137 cases in the FAERS database through April 1, 2014. The patients’ average age was 45.
Seventeen percent of the group developed tumors in locations not exposed to the sun, and nine died.
The researchers said the database contained only about half the information it should have, such as tumor site, patients’ family history of cancer, and earlier immunosuppressive treatment.
Fifteen percent of the cases in the FAERS database were based entirely on information from the TOUCH database. Seventy-three percent were cases initially reported to FAERS but with TOUCH information added. Thirteen percent of the FAERS cases contained no additional information.
Importantly, there was even less patient information in the TOUCH database than in the FAERS database. Out of eight items researchers believe a database should contain, TOUCH had information on two, on average.
“The existence of the TOUCH Safety Surveillance Program, an FDA-mandated program, did not improve melanoma reporting,” the team wrote.
This shortage of data stymies research into possible links between Tysabri treatment and melanoma, the researchers said. As an example, although the death rates in the databases were low, there was no information about survival in many cases, which could lead to flawed survival estimates.
The investigation noted that patients received a wide range of Tysabri doses before they were diagnosed with melanoma. While some received only one or a few injections, others had been treated for a long time.
These observations do not seem to support a link between Tysabri and melanoma, the team said. “A longer therapy duration would be expected if natalizumab caused melanoma via an immunologic pathway, unless existing nevi [lesions of the skin or mucus tissue] were already premalignant lesions,” the researchers wrote.
But other information the team found seemed to suggest a Tysabri-melanoma link. For example, the average age of melanoma patients was much lower than that reported in the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) database. The average age in the institute’s database is 63, compared with 45 in the FAERS database and 41 in cases in academic journals.
In addition, many patients developed tumors in unusual places not exposed to sunlight. Finally, the low melanoma death rate in Tysabri-treated patients differed from that seen in the general population. All these factors suggest that melanoma after Tysabri treatment could differ from other types of melanoma, the researchers argued.
While the molecular workings of Tysabri might promote melanoma growth, studies so far have not found a relationship between the drug and this cancer. In fact, some studies suggest that MS patients, in general, have a lower risk of melanoma than others.
The team said more information on patients could give researchers a better understanding of the potential relationship between Tysabri and melanoma. The implication was that the standard of reporting in the FAERS and TOUCH databases could improve.
To minimize the risk of patients who receive Tysabri developing melanoma, the researchers offered a number of suggestions for IV centers, physicians, patients, and educational programs. For instance, they suggested that all patients should have a skin examination before the start of treatment, and regular physical and skin exams while receiving Tysabri.
While noting that risk of infection and the development of tumors can occur with all immunosuppressive treatments, the team said more studies are needed to explore the risk of Tysabri-treated patients developing melanoma.