FAQs about Vumerity
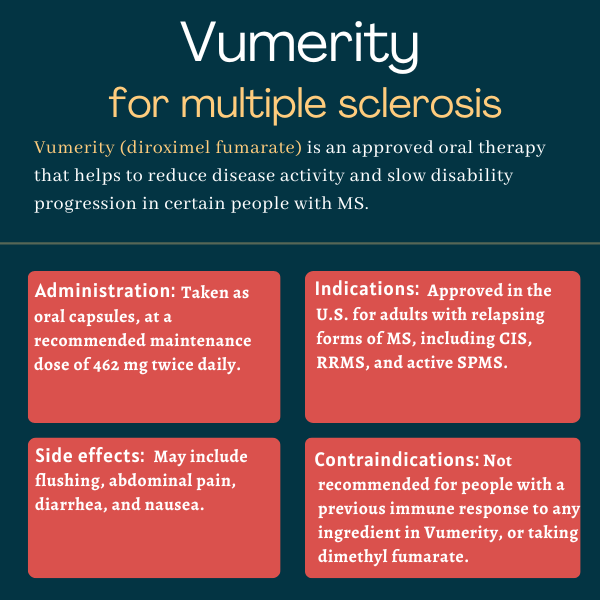
Vumerity was approved by the U.S. Food and Drug Administration in October 2019 for treating adults with relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting MS, and active secondary progressive MS.
There aren’t yet adequate clinical data on the use of Vumerity in pregnancy, but animal studies suggest it may cause fetal harm. Clinical data on the use of Tecfidera (dimethyl fumarate) — which has the same active ingredient as Vumerity — during pregnancy didn’t find any increased risk of adverse effects on the mother or fetus. Patients who become or plan to become pregnant while using Vumerity should discuss it with their healthcare team.
While alcohol may not need to be completely avoided, patients should not drink alcohol at the same time they take Vumerity, as it could interfere with the medication’s absorption. Patients should discuss with their healthcare providers when it is safe to drink alcohol and how much can be consumed in their particular case.
Every person responds differently to a given treatment, and there is no standard timeline for how soon Vumerity is expected to start working. Patients should ask their healthcare providers how Vumerity may help in their particular case.
Weight gain and hair loss were not reported in clinical trials as side effects of Vumerity, but there are reports of hair loss in people who received the therapy’s active ingredient in real-world settings. Patients who experience unanticipated effects from treatment should discuss them with their healthcare team.
 Fact-checked by
Fact-checked by