Botox (onabotulinumtoxinA) for multiple sclerosis
What is Botox for MS?
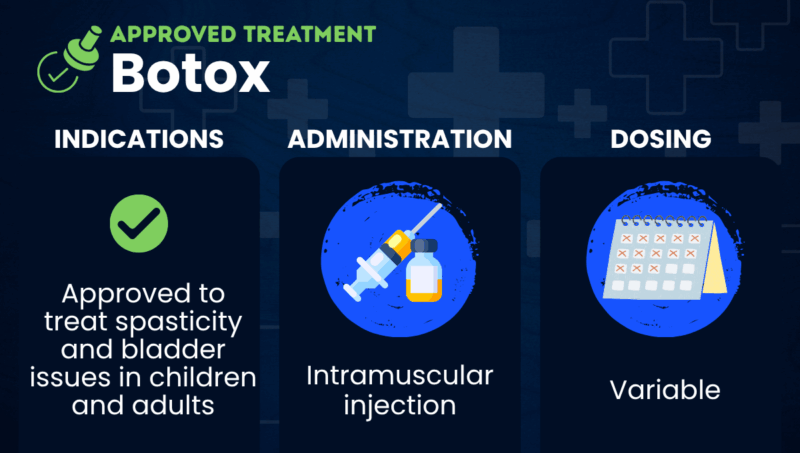
Botox (onabotulinumtoxinA) is an injectable treatment approved to ease spasticity, a common symptom of multiple sclerosis (MS). Spasticity is when muscles are abnormally stiff and tight, and prone to involuntary spasms.
The therapy is also approved for neurogenic detrusor overactivity (NDO), more commonly known as overactive bladder, associated with a neurological condition such as MS. NDO occurs when nerve damage causes the detrusor muscle around the bladder to be overactive, which results in urinary incontinence and reduced quality of life.
Marketed by Abbvie, Botox is given via intramuscular injections, or into the muscle.
The medication contains a powerful toxin that blocks the release of a chemical messenger called acetylcholine from nerve cells. Under normal circumstances, acetylcholine from nerves binds to receptors on muscle cells, which prompts the muscles to contract.
By blocking its release, Botox reduces the signal that makes muscles contract, which can help to ease symptoms, such as spasticity and NDO, that are caused by overactive muscles.
Although Botox can ease these symptoms of MS, it does not alter the progression of the disease.
Botox is approved in the U.S. for several other health conditions, including chronic migraine, strabismus (when one eye is turned in a different direction from the other), eyelid twitching, and excessive sweating. The medication is also used for cervical dystonia, a condition characterized by spasms in the neck muscles, and in cosmetic applications.
Therapy snapshot
| Brand Name | Botox |
| Chemical name | OnabotulinumtoxinA |
| Usage | Used to ease spasticity and overactive bladder |
| Administration | Intramuscular injection |
Who can use Botox?
Botox is approved in the U.S. for the treatment of spasticity in individuals 2 years and older. It is also approved to treat NDO associated with a neurological condition in people 5 and older, who either do not tolerate or do not respond to first-line treatment.
The medication is contraindicated, or should not be used, for people with:
- a history of hypersensitivity reactions, or abnormal immune responses, including allergic reactions, to any botulinum toxin preparation or to any component in the medication
- an infection at the proposed injection site
- a urinary tract infection or urinary retention, if used for treating NDO.
How is Botox administered?
Botox is given via intramuscular injections that are administered by a healthcare provider. The dosage and the specific muscles that are targeted for treatment will depend on the indication for which it is being used.
For spasticity, the therapy is injected directly into the affected muscles, and the recommended dose varies depending on whether patients are experiencing upper or lower limb spasticity. For NDO, Botox is administered via 20 injections into the detrusor muscle.
For both indications, doses are different for children and adults and should not exceed the recommended dosages, which could lead to muscle weakness and other side effects.
Botox in clinical trials
Botox’s approval in the U.S. for spasticity were supported by findings from nine clinical trials:
- Seven trials enrolled more than 1,100 adults with spasticity due to stroke, who received injections of Botox or a placebo into affected muscles. Six of the studies, including three Phase 3 trials (NCT01153815, NCT00460564, and NCT03261167) evaluated the treatment for upper limb spasticity, while the seventh trial (NCT01575054) tested Botox for spasticity in the lower limbs. Results broadly showed Botox injections led to a significant reduction in muscle tone compared with the placebo.
- Two Phase 3 clinical trials tested Botox in children 2 to 17 years old with spasticity. One trial (NCT01603602) enrolled 234 children with upper limb spasticity due to cerebral palsy or stroke. The other trial (NCT01603628) enrolled 381 children with lower limb spasticity due to cerebral palsy. Similar to the findings from adult patients, results from both pediatric studies indicated Botox led to a significant reduction in muscle tone relative to a placebo.
The approval of Botox for NDO associated with a neurological condition was based on data from three Phase 3 clinical trials:
- Two trials (NCT00311376 and NCT00461292) collectively enrolled 691 adults with NDO due to MS or spinal cord injury, who were experiencing at least 14 episodes of urinary incontinence per week and who had failed to respond to or were intolerant of other medications. Results from the two trials showed Botox significantly reduced the frequency of urinary incontinence episodes and led to significantly greater improvements in quality of life than a placebo.
- The third study (NCT01852045) enrolled 113 children ages 5 to 17 who had NDO due to neurological conditions (mainly spinal cord deformities or injuries) and had not responded to standard medications. All participants were given Botox injections into the detrusor muscle at varying doses, and results showed the now-approved dosage significantly reduced urinary incontinence episodes.
Botox side effects
When used in clinical trials to treat spasticity, the most common side effects of Botox included:
- pain in extremities
- upper respiratory tract infection.
In clinical trials for NDO, common side effects included:
- urinary tract infection
- urinary retention
- white blood cells in the urine, called leukocyturia
- bacteria in the urine, known as bacteriuria.
The prescribing information for Botox carries a boxed warning noting the therapy can spread from the injection area and cause muscle weakness in other parts of the body. If the muscles needed to breathe and swallow are affected, this can be life-threatening.
The risk of these effects is believed to be highest in children, but symptoms can occur in adults with pre-existing swallowing or breathing problems. Patients who experience problems with speech, breathing, or swallowing after being treated with Botox should seek immediate medical attention.
Botox use may be associated with safety issues that warrant additional monitoring, including:
- upper respiratory tract infections and bronchitis (inflammation of the airways) when used to treat spasticity
- urinary tract infections and urinary retention when used to treat NDO.
Multiple Sclerosis News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by