Icometrix Collaborates with Novartis on MSmetrix Software Application, New Data at Upcoming ECTRIMS 2015 Conference
Written by |
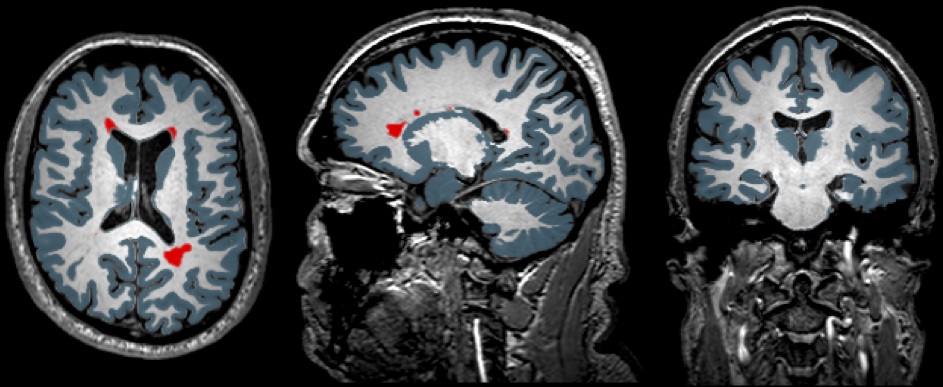
Icometrix, a leader in magnetic resonance imaging (MRI) biomarkers for multiple sclerosis (MS), has recently announced a collaboration agreement with Novartis Pharma AG regarding MSmetrix – a measurement tool especially designed to assess brain volume and atrophy, as well as existing, new or enlarging brain lesions in MS patients.
Monitoring brain changes over time in MS patients helps to assess disease activity and management. MSmetrix uses standard, clinical brain MRI images, and allows a reliable, fast assessment of important MRI biomarkers in MS. The software has been approved for clinical practice in several countries worldwide, including in Canada, Europe and Australia.
“By signing this agreement, MSmetrix will be available for MS clinicians and radiologists, enabling them to better understand the disease status and evolution”, said Dr. Wim Van Hecke, Founder and CBO of icometrix in a press release sent to Multiple Sclerosis News Today. “Providing both measures of brain lesion load and brain volume loss, allows clinicians to use evidence-based and more personalized tools to follow-up individual MS patients”.
The aim of this collaboration is to provide the MSmetrix software to individual MS patients worldwide, offering improved personalized care.
“It is now known that MS is an inflammatory as well as a neurodegenerative disease, and objective parameters to monitor both of these aspects is of crucial importance for the follow-up of individual patients and for making treatment decisions” added Dr. Van Hecke.
Icometrix will be presenting data on the MSmetrix software at the upcoming 31st Conference of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), to be held in Barcelona, Spain, October 7 to 10, 2015, in a poster entitled “MSmetrix, the most accurate untrained method (without needing prior manual segmentations) for longitudinal lesion segmentation in the ISBI Challenge” (poster # 450). The data show the validation of the use of MRI measures through MSmetrix for evaluation of MS patients in a clinical practice setting. Researchers believe that this method will allow evidence-based follow-up of the disease activity and assist on treatment decision-making.
Multiple Sclerosis News Today, from the Bionews Services group, plans to cover the ECTRIMS 2015 Congress and offer the readers real-time data and updates as they are disclosed at the meeting.


