Anokion Planning Launch of Phase 1 Trial of Investigational MS Therapy ANK-700
The U.S. Food and Drug Administration (FDA) has accepted an investigational new drug application (IND) for ANK-700, a treatment candidate for multiple sclerosis (MS) that is being developed by the Swiss company Anokion.
With this approval, Anokion can enter clinical testing and is planning to initiate a multi-center, Phase 1 clinical trial to evaluate ANK-700 in people with MS by the end of the year.
“This IND marks the second clinical-stage program in our pipeline, and we look forward to initiating enrollment in the Phase 1 trial with ANK-700 later this year,” Simon Cooper, chief medical officer of Anokion, said in a press release.
The body’s immune system is responsible for defending it against bacteria, viruses, and other invaders. The processes by which the immune system recognizes possible threats is complicated, but at a simple level, the immune system is basically able to “see” everything as either “self” (a normal part of the body) or as “non-self” (not part of the body).
Anything recognized as “non-self” can trigger a protective immune response in the body, whereas anything identified as “self” should not. This ability of not attacking the body’s own cells and molecules, while still attacking anything recognized as foreign, is called immune tolerance.
Autoimmune diseases like MS are caused by a failure of immune tolerance; by definition, autoimmune diseases occur when the immune system attacks the body’s own molecules or cells.
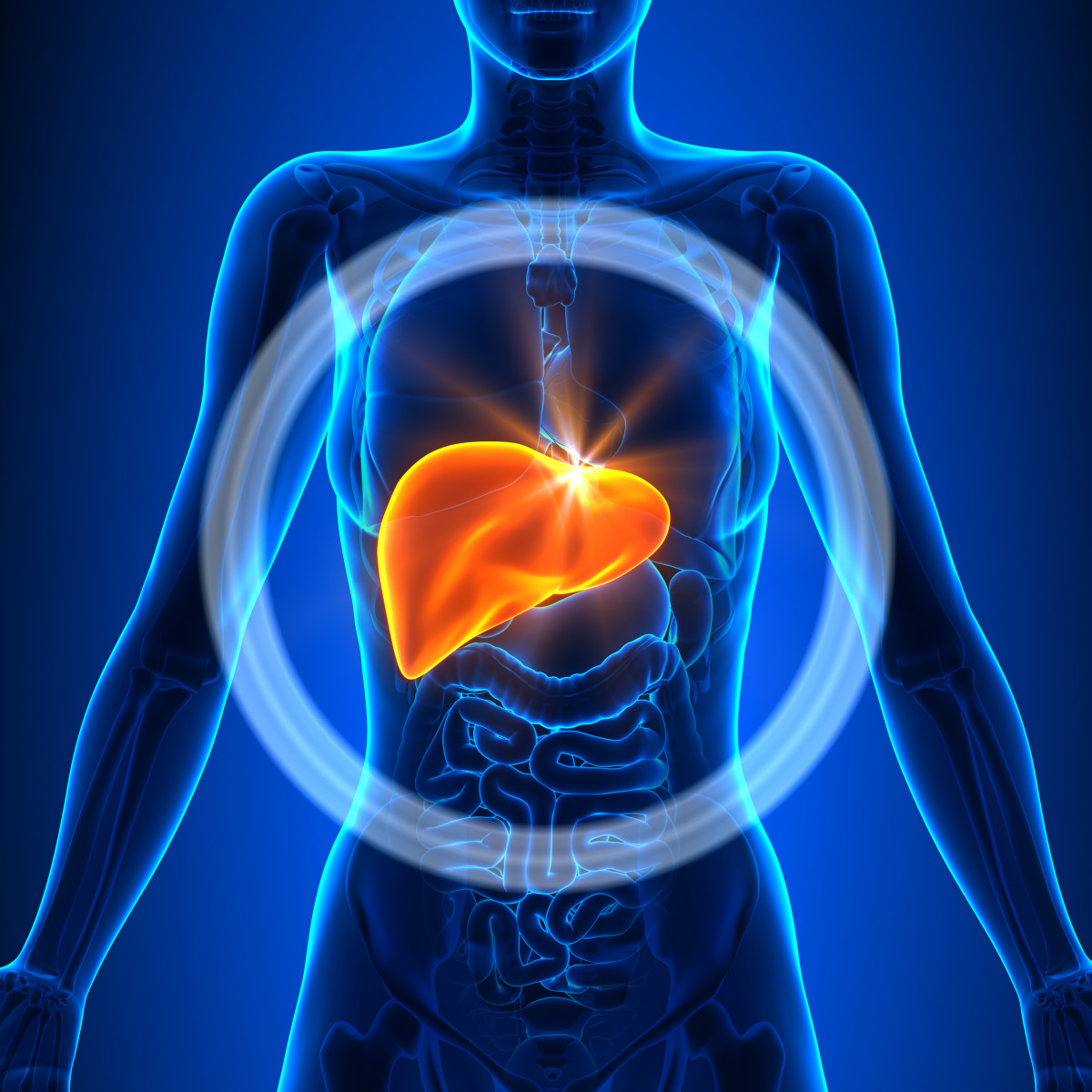
ANK-700 was designed to “re-educate” the immune systems of people with MS, in order to restore tolerance.
The investigational therapy accomplishes this by modulating immune tolerance-associated processes in the liver. Specifically, ANK-700 is thought to affect a subset of immune cells called T-cells, which are believed to be involved in the development of MS.
“Anokion’s approach harnesses the liver’s natural tolerance pathway to modulate the harmful inflammatory immune response in antigen-mediated autoimmune diseases. In these diseases, T-cells mistakenly attack specific self-antigens as if they were foreign, disease-causing pathogens,” Anokion states on its website.
Of note, antigens are molecules capable of triggering an immune response.
In the liver, once cells are exposed to Anokion’s engineered proteins that express the antigen that triggers the harmful immune response — in the case of MS, these antigens are part of the central nervous system (CNS) — “the body no longer mounts an immune response toward whatever tissue expresses the antigen.”
In other words, the therapy “teaches” the immune system to not attack the body’s own molecules and recognize them as “self,” while leaving the rest of the immune system untouched.
According to Anokion, ANK-700 has shown effectiveness in preclinical models of MS, including studies in mice and non-human primates, as the therapy was able to prevent or reduce disease activity.
“By harnessing the natural immune tolerance pathways in the liver, ANK-700 aims to re-educate the immune system with a potentially transformative approach that induces antigen-specific tolerance to CNS [central nervous system] autoantigens and treats neuroinflammation,” Cooper concluded.






