First healthy participant dosed in Phase 1 trial of IMP761 for MS
The study is expected to recruit 49 healthy adults in the Netherlands
Written by |
A Phase 1 clinical trial that’s testing IMP761, an experimental treatment for multiple sclerosis (MS) and other autoimmune conditions, has dosed its first healthy participant, the therapy’s developer has announced.
Immutep received regulatory clearance to start the first-in-human trial of IMP761 in the Netherlands about a month ago. The study, which is expected to recruit 49 healthy adults at a single site in Leiden, will evaluate the therapy’s’s safety and pharmacological properties. Safety data are expected by year’s end, and information on its pharmacokinetics and pharmacodynamics by the first half of 2025. A therapy’s pharmacokinetics refers to its movement into, through, and out of the body, whereas pharmacodynamics describes a treatment’s effects on the body.
MS is an autoimmune disease in which the myelin sheath that protects nerve fibers in the brain and spinal cord is wrongly attacked by the immune system. This leads to a wide range of symptoms that includes muscle weakness, fatigue, and changes in sensation.
While different types of immune cells participate in the inflammatory attacks that mark autoimmune diseases, including MS, most of these conditions feature the excessive activation of self-reactive immune T-cells.
What is IMP761?
IMP761 is an antibody-based therapy that activates LAG-3, a receptor protein on the surface of T-cells. In healthy people, this receptor helps prevent T- cells from becoming overly active and attacking healthy tissue. By activating LAG-3, IMP761 should boost LAG-3’s “brake” function, suppressing the activity of self-reactive T-cells and restoring immune balance in people with autoimmune diseases.
Studies have shown that activating LAG-3 suppresses the growth and activation of lab-grown human T-cells and reduces the production of pro-inflammatory molecules by lab-grown immune cells from people with juvenile idiopathic arthritis, an autoimmune condition marked by joint inflammation and pain.
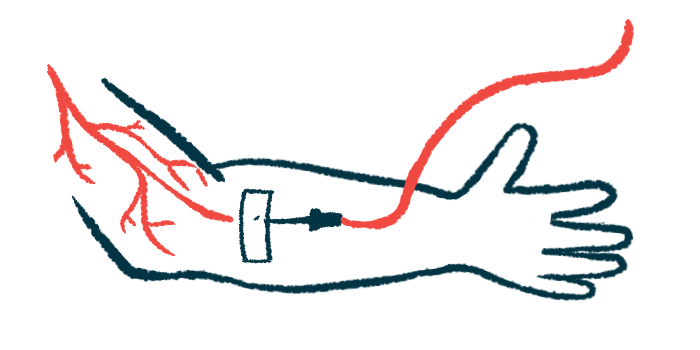
IMP761 is administered directly into the bloodstream. Before it can be tested in people with autoimmune diseases like MS, it must be tested in healthy volunteers. The ongoing two-part Phase 1 trial will assess the safety and pharmacological properties of single and multiple ascending doses.
In the single dose portion, participants will receive a single infusion of either IMP761 at increasing doses or a placebo. In the multiple dose portion, participants will be given multiple infusions of either the therapy at increasing doses or a placebo for a certain period. The study will be double-blinded, meaning neither the researchers nor participants will know who receives the therapy and who gets the placebo.
To assess IMP761’s pharmacodynamics, the Centre for Human Drug Research, which is running the trial, will implement a unique immunization test specific to T-cells that will let researchers evaluate the treatment’s effect at the early stages of clinical development.