FAQs about Tysabri in MS
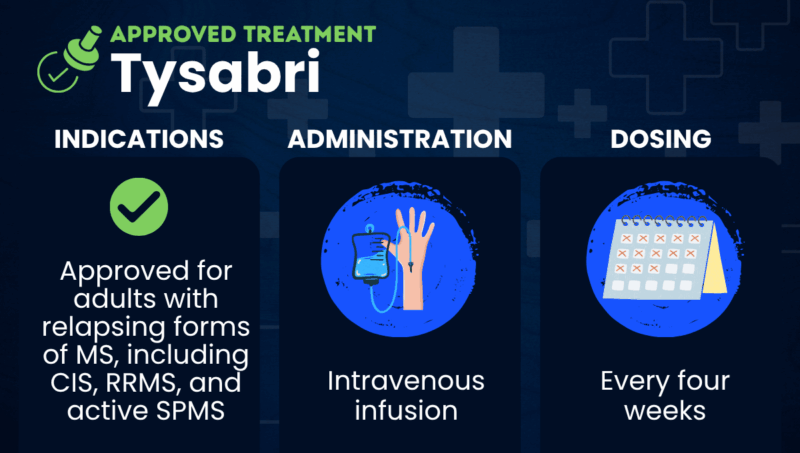
Tysabri was approved by the U.S. Food and Drug Administration in November 2004 to treat relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting MS, and active secondary progressive MS.
In the AFFIRM trial, which tested Tysabri in more than 900 people with relapsing forms of multiple sclerosis, the therapy began to demonstrably reduce the disease activity as soon as two months after starting treatment. However, as every person is unique, patients are advised to discuss with their healthcare team how the medication may help in their specific case.
While it is not yet known if Tysabri can cause birth defects, there have been reports of low blood cell and platelet counts in infants who were exposed to Tysabri during pregnancy. Those who become pregnant or plan to while on Tysabri should discuss the potential benefits and risks with their healthcare providers as early as possible.
Tysabri is not known to interact with alcohol. However, both Tysabri and alcohol can affect the liver, so drinking while on treatment may increase the risk of liver damage. Alcohol may also worsen certain multiple sclerosis symptoms, so patients should always discuss safe alcohol consumption with their healthcare provider.
Hair loss has not been reported as a side effect of Tysabri in multiple sclerosis clinical trials, but some people have experienced an increase in body weight. There are also reports of patients experiencing a decrease in body weight. Patients who experience unanticipated effects after starting treatment should talk to their healthcare team.
 Fact-checked by
Fact-checked by