Severe Demyelination in Non-MS Patient After TNF-α Blocker Treatment Detailed in Study
TNF-α blocking drugs, such as infliximab, surprised investigators when their use in people with multiple sclerosis (MS) actually triggered demyelination. In a case report published in the journal Neuroimmunology & Neuroinflammation, Vanderbilt University Medical Center researchers reviewed an aggressive demyelinating event in a non-MS patient treated with TNF-α blockers.
TNF-α blockers are used to slow the progression of autoimmune diseases with an inflammatory component, such as inflammatory bowel disease (IBD) and rheumatoid arthritis (RA). Based on positive treatment results in both patient groups, clinical trials of drugs targeting TNF-α signaling in multiple sclerosis (MS) patients were commenced — and prematurely shut down. MS patients reacted to the drugs with earlier and more frequent exacerbations, and TNF-α blockers were contraindicated for the disease.
Demyelinating events in people with no previous MS-related history have also been reported in scientific journals. The pathological findings in these patients, upon clinical, laboratory, and radiographic review, resemble MS pathological patterns. The death of the patient presented in this case study allowed for a histological confirmation of demyelination following TNF-α blocker treatment.
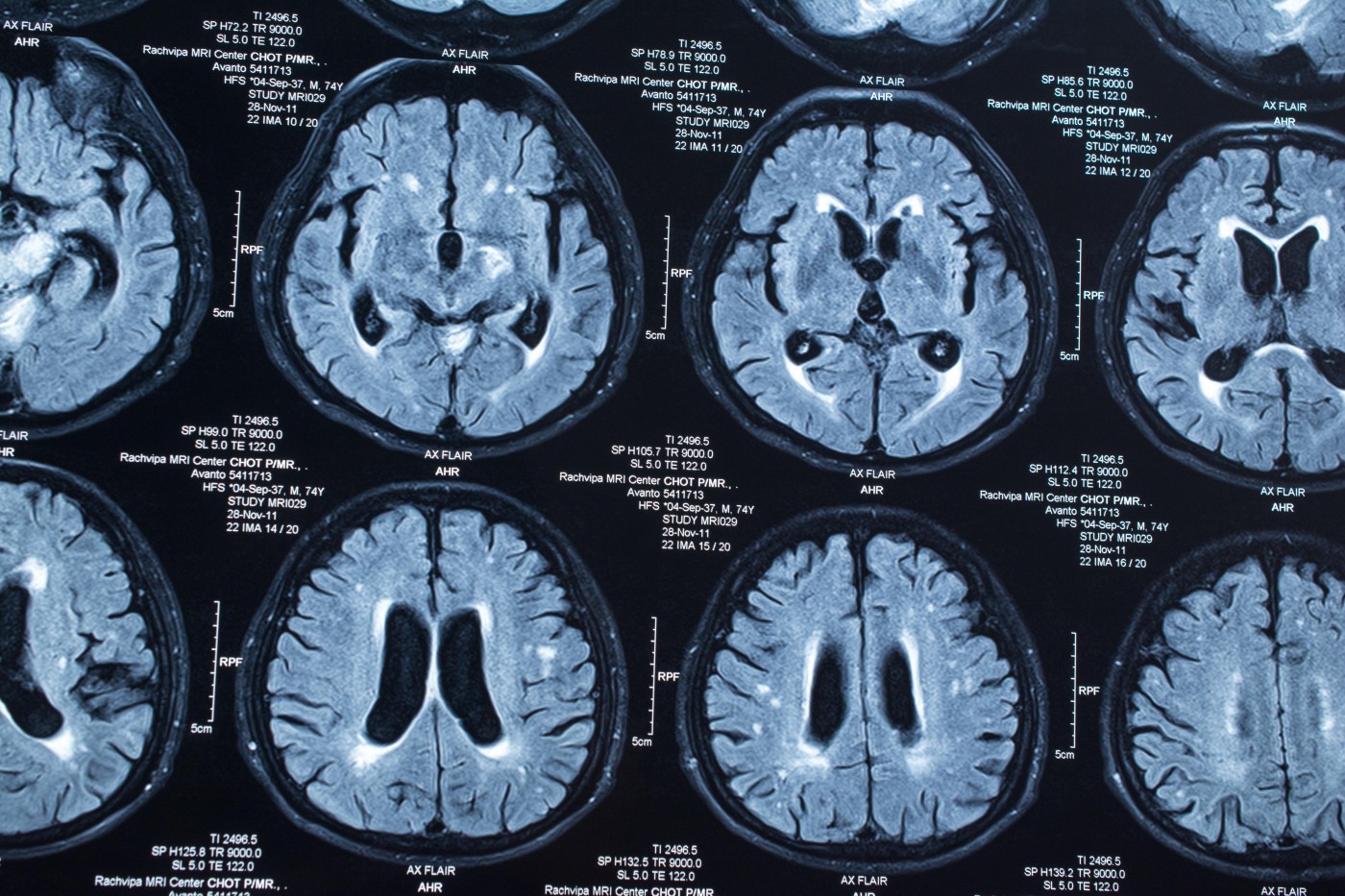
As reported in “Autopsy-Proven Demyelination Associated With Infliximab Treatment,“ a 57-year-old man treated with TNF-α blockers for four months arrived at a hospital with obvious deterioration in brain function, including facial weakness and ataxia. He had also developed a rash. Brain magnetic resonance imaging (MRI) scans showed lesions suggestive of demyelination in the brainstem and in parts connecting the brain stem with the cerebellum. Lesions in deep brain structures, such as the striatum, and in superficial cortical tissue were also found.
Clinicians could find no support for a microbial cause to the brain lesions, and a biopsy of the rash suggested autoimmune mechanisms, toxicity, or hypersensitivity reactions, or disease caused by virus or the bacteria Rickettsia.
The lesions progressively worsened. Although the man had initially responded to aggressive steroid treatment, he died following a multi-drug-resistant infection with Klebsiella pneumoniae. A brain autopsy confirmed a process of acute demyelination, mainly involving white matter.
Previous studies on demyelination following TNF-α treatment present with various clinical signs of demyelination as well as skin reactions, the authors noted. In most patients, neurological complications are reversed upon cessation of TNF-α blockers, but an estimated 25 percent develop MS despite discontinuation.
Researchers are still in the dark about how TNF-α blockers cause demyelination. Several mechanisms have been suggested, such as the possibility that prolonged exposure to TNF-α antagonism increases the immune T-cell response or alters the cytokine profile — events that could favor demyelination. These are, however, only hypotheses and scientists are still searching for the cause of TNF-α treatment-related MS.






