Astrocytes Can Turn Aggressive and Kill Neurons, Potentially Groundbreaking Study Says
Written by |
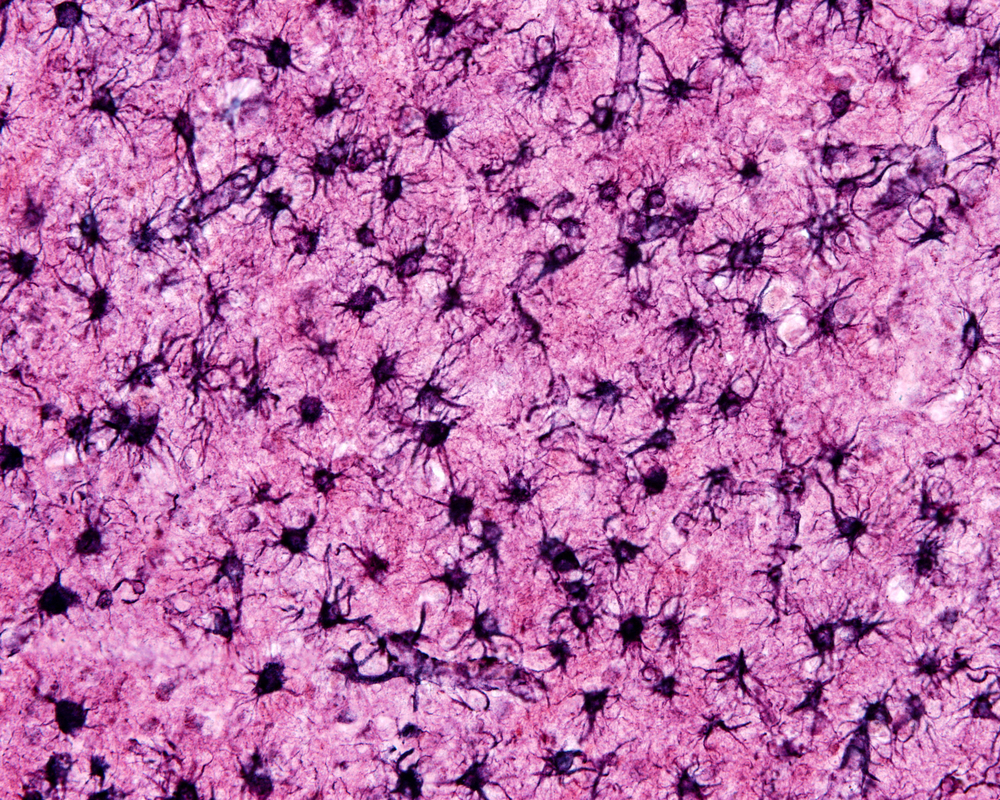
In what may be one of the most significant discoveries in neurodegenerative disease, researchers have found that brain cells, called astrocytes, contribute to killing neurons and myelin-forming oligodendrocyte cells, which may drive neurodegenerative diseases such as multiple sclerosis (MS).
Experiments indicate an aggressive astrocyte type kills cells by secreting a yet-unidentified factor. Researchers are working to identify the secreted molecule, as well as to determine how to stop good astrocytes from becoming bad. Doing so may help them interfere with the neurodegeneration that occurs in MS and other conditions.
The study that explains the discovery, “Neurotoxic reactive astrocytes are induced by activated microglia,” was published in the journal Nature.
“We’ve learned astrocytes aren’t always the good guys,” Ben Barres, MD, PhD, the study’s senior investigator, said in a press release. “An aberrant version of them turns up in suspicious abundance in all the wrong places in brain-tissue samples from patients with brain injuries and major neurological disorders from Alzheimer’s and Parkinson’s to multiple sclerosis.”
Barres called the finding the “the most important discovery my lab has ever made.”
Astrocytes are key players in forming and modulating connections and communications between neurons. In the absence of disease or injury, the cells are referred to as “resting astrocytes” — a somewhat contradictory term given the huge amount of work they do.
Under certain circumstances, astrocytes change their appearance and behavior, becoming “reactive.” The star-shaped cells have been the focus of extensive research efforts, and in 2012, the research team at Stanford University School of Medicine discovered two types of reactive astrocytes. The cells are formed when exposed to different factors. Those that researchers called A1 developed when resting astrocytes were exposed to components simulating a bacterial infection. A2 cells, in contrast, emerged after oxygen deprivation, which can occur during a stroke, for example.
A2 astrocytes have regenerative properties, but the A1 type spews out large amounts of pro-inflammatory factors.
Since that discovery, the team has worked to learn how A1 astrocytes are generated and what they do. Researcher now know that resting astrocytes turn into A1s when another cell type — microglia — is activated.
Microglia reacted to the bacterial components that researchers used in the earlier experiments.
The team analyzed the compounds the microglia released and concluded that a mix of the three factors — TNF-alpha, IL-1-alpha and C1q — was needed for A1 cells to form. The three inflammatory mediators are only secreted by microglia in the brain, ruling out the possibility that other cells might be involved in A1 cells’ formation.
Next, researchers examined the properties of A1 astrocytes using a lab-grow nerve cell model. The cells, called retinal ganglions, grow well in a lab dish, but only if they are accompanied by astrocytes. The team replaced resting astrocytes with A1 and saw that the number of synapses — nerve cell connections — was drastically reduced, and those that were present did not work well.
In examining other aspects of astrocyte-synapse interactions, the team found that A1 astrocytes were not good at pruning unnecessary connections.
The most far-reaching finding arose from another set of experiments. When the team took the broth in which they kept A1 cells and added it to nerve cells in increasing concentrations, nearly all the retinal ganglion cells died. The same thing happened when they exposed other types of neurons and oligodendrocytes to the A1-derived liquid.
That suggested the cells secrete a substance that is toxic to neurons.
Other experiments confirmed that A1 astrocytes kill neurons that have had their axons severed. (When a cell loses its axon — the fiber carrying information out — the entire cell dies.) The team found that by blocking the factors that turn good astrocytes into the aggressive A1 type, they could save the cells from a certain death.
In a final set of experiments, the team examined the brains of MS patients, and those of people who died with amyotrophic lateral sclerosis (ALS), and Alzheimer’s, Parkinson’s, and Huntington’s diseases. They noted large numbers of A1 astrocytes in the areas ravaged by disease.
“We’re very excited by the discovery of neurotoxic reactive astrocytes, because our findings imply that acute injuries of the retina, brain and spinal cord and neurodegenerative diseases may all be much more highly treatable than has been thought,” Barres said.


