FTC Challenges Amniotic Stem Cell Treatment Clinics’ Claims
Written by |


“Deceptive” and “false” are two words used by the U.S. Federal Trade Commission (FTC) to describe marketing claims by two stem cell treatment clinics in California.
According to an FTC complaint, the clinics had been advertising that they were using amniotic stem cell therapy to successfully treat serious diseases, including multiple sclerosis, autism, stroke, and heart attacks. The claims included one advertisement saying their treatment restored sight for a 101-year-old woman who had been blind for seven years.
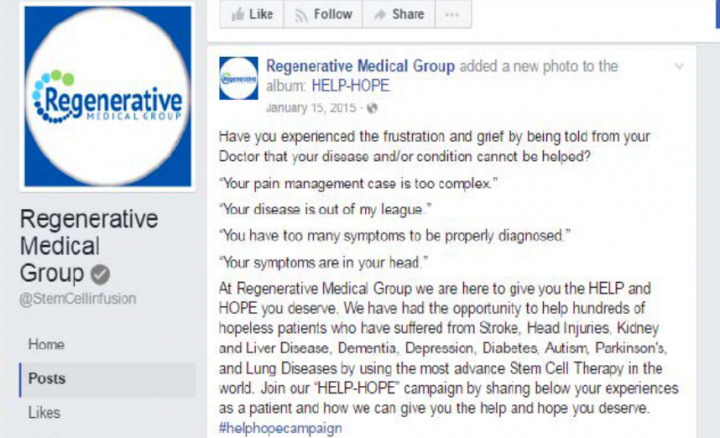
Screenshot of a Facebook post included in the FTC complaint.
A promotional letter included in the complaint claims: “Lives are being saved, the blind see, the crippled walk and the patients with heart, lung, kidney and nerve diseases can alter the course of their suffering with a simple therapy. …”
Discuss the latest research in the MS News Today forums!
There’s no proof that it works
The FTC says the clinics treated patients with stem cells derived from the amniotic fluid of women who had cesarean sections. (Amniotic stem cell treatment shouldn’t be confused with autologous stem cell transplants, in which stem cells are removed from a patient’s body then replaced after chemotherapy.)
In its complaint, the FTC charged that there is no proof that any of the treatments provided by the clinics worked. “The representations … are false or were not substantiated at the time the representations were made. In fact, many of these diseases are considered to be currently incurable by medical professionals,” the agency said.
The FTC demands a payback
An FTC news release stated:
“Dr. Bryn Jarald Henderson, D.O. and the two companies he owns and operates, Regenerative Medical Group and Telehealth Medical Group, earned at least $3.31 million offering stem cell therapy between 2014 and 2017. Initial stem cell therapy injections ranged from $9,500 to $15,000, with patients encouraged to undergo multiple treatments. Follow-up ‘booster’ treatments cost between $5,000 and $8,000 each.”
In the proposed settlement, Dr. Henderson and his companies are prohibited from making health claims “unless the claims are true and supported by competent and reliable scientific evidence.” The settlement requires the companies to pay the FTC a partially suspended fine of $3.31 million, the approximate amount the commission says the company earned between 2014 and 2017. Of that, $525,000 would be set aside for patients who may have been harmed by the treatments.
The moral of this column, of course, is patient beware. If a treatment sounds too good to be true with unverified claims of success, it’s probably wise to steer clear.
You’re invited to follow my personal blog at www.themswire.com.
***
Note: Multiple Sclerosis News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The opinions expressed in this column are not those of Multiple Sclerosis News Today or its parent company, Bionews Services, and are intended to spark discussion about issues pertaining to multiple sclerosis.



Tom Harrison
Does his mean there would need to be a 3 phase FDA study?
Ted Heckert
The FDA is currently working with two Dr.s in Winchester Va tracking the success of their treatments with umbilical stem cells. I know that my mother-in-law was treated with a fair amount of success in a few short weeks. Drug manufacturers do not want stem cell success as it will cut deep into their pockets. My mother-in-law no longer takes pain medication. If it were not feasible why would have a pharmacist and friend have had the treatment? There are documented success with stem cell treatment. You are just getting the negative side. Thank you for taking the time to read this.
Ed Tobias
Hi Ted,
To be clear, the clinics being investigated by the FTC are offering treatments using stem cells from a woman's amniotic fluid. This is different from stem cells obtained from the umbilical cord, or Hematopoietic Stem Cell Transplantation.
https://www.nationalmssociety.org/Research/Research-News-Progress/Stem-Cells-in-MS/Bone-Marrow-Stem-Cell-Transplant-%E2%80%93-HSCT
Ed
Dorothy
What the FTC should be investigating is the harvesting of stem cells derived from cord blood. I have read about a stem cell clinic in Panama that harvests stem cells from cord blood. You probably already have heard about this.
Ed Tobias
Hi Dorothy,
I'm not sure that I understand your concern. The FTC is investigating unproven, and allegedly false, claims that stems cells taken from a woman's amniotic fluid can be used to successfully treat a number of diseases. That's different from the stem cells that are obtained from cord blood. Those are used for hematopoietic stem cell transplantation procedures. You can read more info about HSCT here: https://www.nationalmssociety.org/Research/Research-News-Progress/Stem-Cells-in-MS/Bone-Marrow-Stem-Cell-Transplant-%E2%80%93-HSCT
Amy Householder
I went to the clinic in Panama for the cord blood stem cells. It was very expensive and did not work