Are the Benefits Worth the Risks of Lemtrada?

My neurologist calls Lemtrada “HSCT lite.” She says that not only is the drug able to reduce exacerbations and limit the overall progression of multiple sclerosis, it’s actually reversed some symptoms in some of her patients. I guess I’m going to find out if she’s right about Lemtrada because I’m getting ready to start using it.
Lemtrada Targets MS-related Cells
Lemtrada, which carries the generic name alemtuzumab, was originally approved by the U.S. Food and Drug Administration, at a much higher dose and under the brand name Campath, to treat B-cell chronic lymphocytic leukemia. The drug is a humanized monoclonal antibody which seeks out and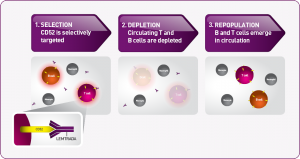 destroys specific immune cells that carry a protein called CD52. Many of these are believed to be the white blood cells that attack the central nervous system in people with MS. After those cells are removed, the body manufactures new white blood cells that have different properties, i.e., beneficial helper cells rather than the destructive cells that are programmed to attack myelin. Thus, the similarity to stem cell replacement therapy. That therapy uses chemotherapy to destroy most of the body’s immune system cells. Then, fresh stem cells are infused into the body which, over time, create new white blood cells.
destroys specific immune cells that carry a protein called CD52. Many of these are believed to be the white blood cells that attack the central nervous system in people with MS. After those cells are removed, the body manufactures new white blood cells that have different properties, i.e., beneficial helper cells rather than the destructive cells that are programmed to attack myelin. Thus, the similarity to stem cell replacement therapy. That therapy uses chemotherapy to destroy most of the body’s immune system cells. Then, fresh stem cells are infused into the body which, over time, create new white blood cells.
Unlike other disease modifying therapies (DMTs), which require injections every few days or monthly infusions, 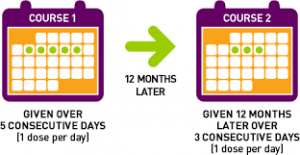 patients receiving Lemtrada are treated once a year, for two years. The first year the drug is infused daily for five days in a row, on an out-patient basis. Each infusion takes about four hours and the patient is watched for another two hours afterward. A year later that process is repeated, but for only three days. And that’s it.
patients receiving Lemtrada are treated once a year, for two years. The first year the drug is infused daily for five days in a row, on an out-patient basis. Each infusion takes about four hours and the patient is watched for another two hours afterward. A year later that process is repeated, but for only three days. And that’s it.
Clinical Trials Show Multiple Benefits
I was attracted to the drug because it’s been shown to improve symptoms as well as halting disease progress. In a study conducted by researchers at Queen Mary University of London, and published in the October 2016 issue of Neurology, nearly half of the patients treated with alemtuzumab (Lemtrada) showed improvements in all seven functions on the EDSS functional scale two years after their treatment began. (EDSS functions include things such as limb movement, numbness, and speech and bladder and bowel functions). Those results, the researchers write, “suggest that such disabilities may often be reversible (at least partially) in patients with active RRMS if they receive suitable therapy, irrespective of the type of baseline functional deficit.” My own neurologist tells me that one of her wheelchair-bound patients has regained some walking ability. Naturally, I was interested in exploring it as a therapy.
There Are Risks
But, this treatment is not without serous risks. They include autoimmune problems that could result in severe bleeding or kidney problems, serious infusion reactions, and an increased chance of getting certain types of cancer. Patients receiving Lemtrada are required to have their blood and urine tested monthly, and they’re monitored very carefully for as long as four years.
Those considering Lemtrada will have to have a discussion with their neurologist, and then determine whether the possible benefits of this treatment outweigh the risks. For me, the balance falls on the side of the potential benefits. My first series of infusions is scheduled for the first week in December, and I’m sure that I’ll be writing at least one column that week with an IV stuck in my vein. Stay tuned.
[You can read more of my columns on my personal blog: www.themswire.com]
Note: Multiple Sclerosis News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The opinions expressed in this column are not those of Multiple Sclerosis News Today, or its parent company, Bionews Services, and are intended to spark discussion about issues pertaining to multiple sclerosis.






Joan Quilter
I think you are very brave. My daughter was diagnosed in 1997, and is, now, bedridden with severe disabilities. I suppose her MS, now, would be called SPMS, for lack of a better label. Lemtrada sounds great, but the risks are too scary for her. She's not ready to take the leap.
Ed Tobias
Hi Joan,
Thanks for your comments. I'm not brave, though I am nervous. I was on Tysabri for a couple of years and that drug, also, had the possibility of having some serious side effects to consider. I have a lot of trust in my neurologist, who I've been seeing for over 15 years, so that makes these kind of decisions much easier.
I hope that your daughter will find the right drug for her treatment and that she will do better in the future.
Ed
Kyle
Joan I hope that Lemtrada has had a positive effect for your daughter. This disease is awful in so many ways. Ed I’m going through the same thing as you had. I have been on tysabri for 7 years since I was first diagnosed until I tested positive for JC virus in my last blood work. I trust my neurologist very much but can’t help but worry I guess when it comes to such a big switch especially when I see all the possible negative side effects.
Ed Tobias
Hi Kyle,
Two things. First, as my JCV numbers began to increase my neuro switched me from a monthly Tysabri infusion to one that was every other month. That bought me another couple of years on that med.
Also, my column about risks vs benefits was written before I began Lemtrada treatments. I'm now a year post Round-2 of Lemtrada and glad that I was given the treatment. Though there are certainly some serious side effects to consider, and at the start it can be a roller coaster ride particularly regarding energy level, think I'm walking a little better, I'm sharper cognitively and I'm sure that my bladder and bowel functions have improved.
Over the past two years I've written several columns about my Lemtrada experience. You can find them if you search on this website for Lemtrada, Tobias or The MS Wire.
Ed
KATHY FRAZIER
Joan, I too have MS & had it since 1993. I am ready to do this medicine. I now can't walk with out my walker. I get very tired when I get ready to go out. In a about a another year I won't be able to do anything for myself. My husband has to do everything from cleaning house, wash clothes to cooking. I'm tired & I'm ready to do something. Yes, I'm afraid but I know if I don't do something I might regret it later.
Mario
I know took 3 cycles of lemtrada and it's well worth the risk especially if I was a patient that is bedridden which I am not. I have seen nothing but good since I took my first infusion
S
How have you seen nothing but good? I'm very interested in this drug.
Ed Tobias
This column is a couple of years old. Please look at my MS Wire columns that are more current to see my experiences using Lemtrada. For me the benefits have far outweighed the risks.
Ed
Sharon Bosch
Hi Ed,
I have received my second round of Lemtrada this year in early February. My first round last year was the best thing I could have ever done. For the first time in 20 years I could feel my right hand, balance with ease, my legs did not feel heavy anymore and I was more awake than I had been since I was diagnosed with MS. By the end of November last year, the effects started to wear off and I was having another flare up. I didn't panic though because I knew I would be having my second round of treatment in February. I was actually very excited about it.
Unfortunately, I am not seeing anywhere the same results that I did with the first round. I am so disappointed right now and trying my hardest to stay positive. I have lost feeling in my right side again and am extremely exhausted. To the point that it's effecting my job. I just received my 3rd day of the solumedrol treatment for this flare up. The nurses told me it could be from my T cells and B cells growing unevenly right now which causes a flare up. If things still don't improve, the doctor will have me go for round 3 of the Lemtrada in the beginning of next year.
My question to you is, have you heard of anyone else having this result? I'm trying very hard to stay positive but I am really let down this year.
Ed Tobias
Hi Sharon,
I'm very sorry to hear about your disappointing results. Though the majority of people I've heard about are doing well after two rounds I know that many haven't shown true improvement until after round three.
I'm sure you've heard, many times, that we're all different. Our MS is different and our response to different meds is different. I'm doing very well, at least in my opinion, 14 months following round 2 and I've written several columns about my journey. If you use the search function of this web site for my name and Lemtrada, or for the MS Wire and Lemtrada, you should be able to find them. Or, just search for The MS Wire and you'll have interesting reading for days :-).
Don't give up. It may take a third round for you. Or, it may even take a switch to a different DMT. There's nothing wrong with being let down and disappointed. But in the nearly 40 years I've lived with MS I've learned that mental attitude plays a role in getting the most out of a treatment. Don't fail to use mental toughness to your advantage.
Please let me know how things go.
Ed
W Smith
I started Lemtrada 2 years ago and have gone through both the infusions. 4 months after the 1st treatment I began to notice improvement and it hasn't stopped, I told my doctor it felt like waking up. No longer do I need to lean on carts for balance when in a store. I can even jog for very short distances and am hoping to ride a bicycle if things keep getting better. Everyone tells me to slow down and that I might be getting ahead of myself. Lemtrada has given me a 2nd shot if I can just figure out how to make it happen. Exited about life again after 21 years of RRMS.
April D Honeycutt
I was one of the people who was on the study drug Lemtrada before it was approved by the FDA. I think it's a great med for RRMS. March 25,26,27th I will have to have my first infusion since 2005 when I was beginning the study. There are risk, but life is a risk. This disease is no joke!
Chuck kimball
I am in my fourth year of Lemtrada. This is done wonders for me as far as ms is concerned. Sadly to say, I've recently been diagnosed with two different cancers 4 years later and the result of the lemtrada treatment. (Thyroid cancer and HPV cancer) I believe if I was tested for HPV before treatment of Lemtrada my decision might have been different. Now I'm pretty scared to even try to do any type of chemo in regards to this cancer. We all are so different and I honestly believe that it should be a priority that people are tested properly before any decisions of taking lemtrada are made. Lemtrada has given me a great quality of life up to this point of now having to deal with these two cancers. I can only say this to my fellow ms'ers. Do your homework and demand that certain tests are done before you make the decision for this treatment ✌️??
Vikki
I wish you luck in this new path. I was Dx in 1993 and have been on most of the dmt's. I received my first round of Lemtrada in February 2016. I am almost eight months post-Lem and so far, so good! I have no new lesions, and no new MS activity. My biggest advice, hydrate hydrate as much as you can during infusion week, and be as positive as you can!
Ed Tobias
Thanks for the info, Vikki. I'll post progress reports after I start down this road in December.
Ed
Vikki
If you have any questions prior to infusion or after, please feel free to reach out. There is also a wealth of information in the Lemtrada for MS treatment Facebook group. Members are unbelievable with support, and there are several people who have been in the clinical trials as well. Good luck!
Vikki
Ed Tobias
Thanks, Vikki. I was unaware of the Facebook group but I'll be sure to check it out. I also appreciate your kind offer of help.
Ed
Tiffany Maczuga
Hey! I’m about to start on Lemtrada but I’m very nervous. I am 42 and have limited mobility with my left leg. Have you had great results with Lemtrada?
Christina
Hi, my husband is going to Chering Cross on Monday to start Lem. I wish you all the best too, Tiffany :)
Mario
I have had multiple sclerosis since 2000 and I have tried numerous other therapies and nothing has worked for me the way lemtrada has. I just took my third round of Lemtrada in January of 2018 because my neurologist thought maybe it will help with my walking which I am walking without any assistance from a cane, Walker or anyting but hoping you will improve more. Since I took my 1sr infusion of lemtrada I have not had any active lesions or progression. Good luck on your decision
Polly
Hi Vikki,
So nice to hear that Lemtrada has worked for you. I am 10 days away from starting it and am nervous about the side effects and also if it is going to help me with my right leg weakness. I also hear from the blogs that Lemtrada won't be very successful if you have had the disease for a long time. I was diagnosed in 88 after getting tingling and numbness in my right leg. It gives me hope after reading that you have not had any further lesions. Any advice at this point will be well appreciated as I am starting to get cold feet about going ahead with this treatment.
Ed Tobias
Hi Polly,
Hope you don't mind my butting in. I've written several columns about Lemtrada since I wrote the one to which you've responded. My latest appeared just a couple of days ago, following my 9-month anniversary. If you take a look at it, and its links to a few of the others, I think you'll get a good picture of the pluses and minuses for me...and the pluses outweigh the minuses. As for whether Lemtrada works for someone who was DXed a long time ago, I have you beat. I was DXed in 1980!
Good luck in your decision.
Ed (The MS Wire)
Polly
Thanks for your response Ed. Where can I read the several columns written by you? I would love to read them to increase my confidence about starting Lemtrada. Would also like to get on the Facebook which I hardly ever go on. How does one get on the ms Lemtrada blog? I am so worried about the weakness and low immunity levels we have to face after the week of infusion. How was it for you? I have a 9 year old special needs child who is on the moderate spectrum so I worry about how I would look after him. Would I be able to get on my feet after a couple of weeks post treatment. Any input would be greatly appreciated.
Ed Tobias
Hi Polly,
Sorry. I intended to include a link to my columns on this web site. Here it is: http://multiplesclerosisnewstoday.com/category/the-ms-wire/. If you'll scroll through you'll find the one's regarding Lemtrada. They begin around the date of my first infusion in December of last year.
You'll also find info from Lemtrada patients in this Facebook group: https://www.facebook.com/groups/1723792797845130/. This group is good regarding actual patient experiences but keep two things in mind; not all of these patients know what they're talking about outside of their own experiences, and groups like this tend to hear more from people who have bad experiences than those for whom things are going along well.
Like MS, which is different for each of us, so is Lemtrada.
I tried to do too much, too quickly, not long after my infusion...boosted by the steroid rush. But then it wore off. I drove from MD to FL about a month after the infusion and also caught a bug which resulted in a fever. That left me pretty tired for a couple of weeks around the 5 - 6 week point. I'd suggest that you try to line up someone to help, just in case you get knocked for a loop. You may not need the help but it couldn't hurt to have someone on standby.
Ed
Mario
I stated in other comments that I've had Ms since 2000 I am currently 43 years old and it has been good for me
Pedro da Costa Pinto
Hi Mario,
How are you now?
I'm on exams to start Lemtrada.
Tami
Hi Ed, I am very interested in watching your progress. I also am wanting to try Lemtrada. I was on avonex for 15 years, then recorders for another 4. Had bad side effects from both, so I am now off therapy. My dr. Is still skeptical of Lemtrada so won't prescribe it. I will be relocating to Oregon soon and will be discuss this option with my new dr. So hopefully soon I will get a chance to give it a try. Best of luck Ed
Tom jenner
Hi folks. I've been reading all your posts, the people that are anxious to try lemtrada but are nervous about it. Remind me of myself a yr ago. I'll be starting round 2 in july, honestly. I went through a period that I wanted to cancel the 2nd round personally I felt no better. My walking has gotten worse and the biggest thing I've noticed since starting lemtrada last July 2016 is the Depression I deal with every day. To me the depression has gotten alot worse since starting the lemtrada. Not really sure why. I feel fortunate that I had no real serious side effects. In the round one series But I almost feel like I'm pushing my luck going for round 2. When you sit and read all the things that could go wrong. Yes it's scary. But I was kind of limited to what m.s. drugs I could try because of testing positive for that JV Virus, something I had never heard of. Have any of you been tested for the virus. Anyone test positive for it. If so. Are you limited to drug therapy let me know. Please. Tom
Vikki
Hi Tom,
I've now completed my second round of Lemtrada in March, 2017. I wanted to let you know you are not alone. About three to four months after round one, I started to experience a huge increase in my anxiety levels. I didn't have very bad infusion reactions outside the expected fatigue and joint pain. After going through more thorough thyroid testing and hormone tests, it was found that I had a chemical imbalance from the five days of steroids and taking an anti anxiety medication for a few months took care of the problem. It's also a very normal reaction to not want to put your body through round two. Going into round two I had felt good. Not symptom free, I've had ms too long to repair past damage, but finally stability. But not doing round two is only completing half of the treatment and for me I didn't want to negate the last year. As to the JC question I am positive. I had high titre levels from the five years of Gilenya I had done prior to Lemtrada. I was advised to discontinue it and I could not go on Tysabri or Tecfidera. I was offered Rituxin or Lemtrada. I chose Lemtrada because if there is a chance of stopping progression I was willing to accept the risks that come with it. I hope you find some stability as well, and if the depression worsens let your doctor know. Being mentally positive is a big part of all of the newer treatments. I have found that I was mentally prepared for round two because I knew what to look out for from last year! Good luck in your decision!
Polly
Hi Vikki
I would love to know how are doing after finishing your 2nd year on Lemtrada. Hope you are out of the depression phase. I am concerned about the low immunity a person goes through post Lemtrada. How long does that phase last?
Vikki
Hi Polly,
I am a little more then six months post round two now. I will be honest, the second round has been a bit more difficult for me than the first. My immune system was very low going into the second round. I have to say though, it is 12:30 am and I just walked into my house after going to see an amazing rock concert with my twenty year old daughter, and handled the strobe lights and could drive us home! I haven't been able to do this in years! How I will feel tommorrow? I don't know but I am just trying to wrap my head around the fact that I did more today than I have been able to do in the past five years at least. For this I will be eternally grateful. My brain, cervical, and thoracic spine all showed many lesions, but no new or enhancing lesions. At 25 years, I cannot ask for better than that! My absolute lymphocytes are a little over 500 which is still low, but all other numbers are back in the normal range. I truly wish you the best in your journey...if you have any specific questions please ask away!
Polly
Hi Vicky
Thanks for replying back. I wanted to ask you how long were you first diagnosed and what area of your body was affected by MS and how do you feel now? You mentioned about having more energy which is great. Any do's and don't before starting the treatment?
Vikki
Hi Polly,
My first symptom of rrms was when I was 17. I went numb on the left side of my body. It lasted about a week and went away on its own. The Neuro at that time never mentioned MS. Then when I was 23 I had optic neuritis. New Neuro (who I still use today as my regular Neuro, not my specialist who prescribed Lemtrada). I was Dx in 1993 so there were no medications for MS at that time. I have been on Betaseron, Copaxone, Avonex, Rebif, and Gilenya before Lemtrada. I have had so many relapses I can't even count in the 25 years I have had MS, and pretty much every area of my body has been touched. My goal with Lemtrada was solely to stop progression. Lemtrada does not reverse past damage, although many people have experienced reversal of symptoms. I don't know how long you have had MS but the earlier you are in the disease process, the better chance of success. There are many does and donts with Lemtrada. My biggest advice to you is do your research, this is not a treatment you can reverse or stop taking because of side effects. If you can, consult with more than one specialist who has experience with Lemtrada. I researched for a year before committing. I wish you luck with your decision! Sorry for the long response!
Wendy Francis
Hi my daughter has just finished roundv1 of Lemtrada last friday all weekend she has severe pains in her hips and knees to the extent she cannot walk. Is this normal as im trying to seek advice no one is getting back to me. Thanks
Ed Tobias
Hi Wendy,
After my first round of Lemtrada I had pain in my hips and thighs. At one point, probably about 5 months post-Round 1, the thigh pain could be shooting. But the hip pain continues for me, even now post-Round 2, but it's hardly noticeable. Your daughter is going to experience a lot of things during this treatment. The column that you read was written before I began Lemtrada. Right now, I'm about ten days past Round 2 of the treatment. I've written several columns about my Lemtrada treatment. If you read them I think you'll get a good picture of what it's like (though everone's experience is a bit different). Just click the "Columns" tab at the top of the website's page and scroll through The MS Wire. Or, search for "Ed Tobias." You'll find them.
There are also a couple of Facebook groups that are made up of Lemtrada patients that you might find useful. With them, however, keep in mind that there can be a lot of incorrect information mixed in with these personal experiences.
Good luck to your daughter.
Ed
Polly
Hi Tom,I am very sorry to hear that Lemtrada didn't work for you and you didn't want to continue with the second year of infusion. I am nervous about starting this treatment in 10 days. My neurologist wanted me be on Lemtrada as I was on Tysabri for a year before getting a positive for JCV. I am furious to know how you are doing now.
Polly
Sorry I meant to say curious
James Mitchell
Hi Tom. I thought I was the only one with increased depression. Like it was just life?? I would like to hear how you are doing,and what you have done to help the situation. I know depression is an MS gig,I just feel a correlation with the infusion. Thank you and God bless.
REESHONDA RENEE OUTLAW
Hi yes I'm was tested positive. Idk what it is exactly .I'm scared of starting lemtrada with all side effects. I also am a diabetic an steroids aren't my friend.
Ed Tobias
Hi,
I've written several columns since I wrote this one. They outline my experience with Lemtrada over the past year.I think you'll find them useful if you search for them and read them. Also, I suggest that you go to Facebook and search for Lemtrada groups. There are a few and they can give you a very good idea of what other Lemtrada users have experienced.
My side effects have been minimal and I've had some improvement in my symptoms.
Ed
Lou
Hi Reshonda
I am about to start Lemtrada, I have been type 1 Diabetic since 1983 and was dx with MS in 2017. It appears to be extremely active so my Neuro has recommended this treatment, did you start Lemtrada?
Paul Koch
I'm 60 years old and was dx'd '06. I was on the 99th Tysabri infusion with no benefit other than slowed progression, as far as I tell. I didn't think of it as bravery, but I had the Lemtrada infusion program done the week of May 16th of this year. I hadn't had so much as a sniffle for twelve years, but all that changed on May 28th. I was warned that there would be a possible 'crash' within a week or so of the completion of the Lemtrada infusion. On the 28th I attempted to use my walker to go to the bathroom, but I was so weak, I could only make one step before falling down. I was unable to get without assistance and it was very difficult to breathe. I was transported by ambulance to the hospital. I was initially diagnosed with pneumonia, but after a few hours I had C-Diff virus, NORO virus, and a UTI. I was in the hospital for nine days; on June 5th transferred to a Rehabilition hospital. I under went physical therapy twice a day, six days a week. When I arrived, I couldn't even stand up, but after about ten days of grueling therapy, I could use the walker again, although I can't travel the distance with it that I could prior to Lemtrada. After three and half months, I was released form rehab on October 12th. I'm going to outpatient rehab a few days a week and do all kinds of exercises at home daily, but it's a struggle everyday. A struggle that I didn't have prior to Lemtrada. I've already decided not to do the second dose in April 2018. This is my experience with Lemtrada. Who knows? Your could be great!
Ed Tobias
Hi Paul,
I'm very sorry to hear about your poor experience after being treated with Lemtrada.
I'm happy to say that I've been doing well as I approach 11 months post round-1. I've written about this in several columns that are more recent than this one.
I wish you the best and hope that you're able to beat whatever knocked you down so hard.
Ed
Rislen
..... thank you for sharing your experiences..
I was diagnosed in 2000
Started with Rebif and had horrible side effects. After that I started with IVIG. This drug is not usually used for MS.
I'm no about 12 years on IVIG. I don't have major problems..... but my neuro wants me to start with Lemtrada, since my brain shows big lesions....
My immun System is quit strong ..... and I'm never sick.... no flu no fever or other classical common diseases....'
And that's why I'm more than afraid to start with Lemtrada..
42 years eating healthy, and building a great immune system..., to now kill it with Lemtrada....
I'm so insecure ?
Ed Tobias
Hi Rislen,
Thanks for your comments. I understand your concerns and I hope that I can point you toward some information that will help you understand the Lemtrada process. First, however, you need to know that Lemtrada doesn't kill your immune system. The treatment targets certain B and T cells, the ones that are thought to be the "rogue" cells that cause your MS. It leaves other immune system cells alone. So, your immune system is compromised for a period of time, but it's not totally destroyed. With good hygiene and some diet restrictions most people seem to be able to avoid catching a bug. Eventually, of course, the cells that are killed should come back, hopefully without the bad characteristics of the original cells.
I've written columns for this web site at 3, 6 and 9 months post-infusion. (I'm now coming up on 11 months). If you use the search function on the site you should be able to find them. You can also search to find news items about Lemtrada that are more technical in nature.
There's also an excellent, patient run, Lemtrada group on Facebook: https://www.facebook.com/groups/1723792797845130/. Be sure to read the drug information that's pinned to the top of the page. Also, browse through the information files that you can access by clicking "files" in the (light blue) left hand column of that page.
I hope this will help make you feel a little more secure. Best of luck.
Ed
Rislen
Ed, many thanks
I will wilk read your columns and join the fb group
Thanks Ed... I'm so scared
Best
Rislen
Ed Tobias
Rislen,
On the FB page there are more than 7,000 MS patients who will watch your back and hold your hand. You're not going into this thing alone.
BTW, I still have my own nervous times. We all do.
Ed
Elaine Nattress
Hello
I have had both rounds of Lemtrada, the second being February 2017. The first year was rough in terms of flare ups of existing symptoms for me but since round 2 I have been generally really well, in fact there are days when I can't remember ever feeling so well. I have had no new symptoms which is what Lemtrada is meant to do.
Just before round 2 my thyroid was underactive for a month or so and then it became overactive. I now have Graves' disease which is one of the potential side effect of Lemtrada and am under the care of an endocrinologist for that. Despite this I have no regrets so far for choosing Lemtrada.
Mike Dee
I'm on Lemtrada for 7 years.
It's excellent and not a bad word to say about it.
Have no fears people; everything comes with potential side effects.
I have had no side effects.
Ed Tobias
Thanks for the comment, Mike.
I'm now just past 11 months post-Round 1 and all is going well for me. I've posted several columns about my journey since I wrote this one.
Ed
CC
I'm considering lemtrada since it doesn't seem like zinbryta is working for me. Should I try tysabri first? I've been on copaxone, rebif, rebif with pulse steroids, tecfidera.
Ed Tobias
Hi Christi,
That's really something for you to ask your neurologist. I can only tell you my experience.I was on Tysabri for several years and it worked very well for me. I moved to another drug whenmy titer score increased to the point where PML was more of a danger.
There are Facebook groups for both drugs where you can read posts from patients who are using those therapies. That may give you a better background when you speak with your doc.
Good luck,
Ed
Ana Ran
Coming in late here-- I was in the Stage 4 trial for Lemtrada, had two infusions a year apart (last one 6 years ago). I had some problems-- got the thyroid disease (but many women my age get thyroid problems anyway, so it might have been a coincidence).
Other than the thyroid meds, I've been off any treatment since 2011. As I said, I was in the clinical trial, and they probably have found ways to limit the side effects somewhat, but it's still a very serious drug.
It didn't just stop the progression for me. It's reversed most of my symptoms. I still have a couple minor issues with my hand and restless legs, but nothing like before. So I'm a success story. Two caveats-- it's a very expensive (VERY) treatment (now it's approved, so insurance should cover much of it), and it takes a week or so each time, and infusions with basically a cancer drug isn't fun for a week. (Then again, it's only a week... not months of chemo, so I counted my blessings.)
Also, there's about a month's time when the immune system is building back, and you could be prone to viruses especially then.
It's a tough decision to make. I didn't have insurance then, and could only get meds through a clinical trial, so I sort of stumbled into it.
There are real dangers, but many of the people in these trials ended up almost symptom-free. My last test, the neurologist said I was "neurologically normal." Yay! But it is such a serious drug.... research a lot first.
Ed Tobias
Thanks for all of the info.
This column was written in Oct. of 2016. In Dec. of 2016 I had my first round of infusions. I'm due for the second round right now but am pushing it back a few months due to some travel.
So far, so good. Was it easy? No, but my side-effects have been minimal. Am I cured? No, but some symptoms seem to be a little better. I've been writing columns about every three months during this journey and plan on writing a 12-month (actually 13-month) report in January.
Happy holidays,
Ed
Mario
I listed above I have just completed my third round of lemtrada and has been all good for me. The only reason I took a third dose is because I have seen such an improvement my doctor thought maybe it would actually improve even more with my walking. I've had multiple sclerosis for 18 years and I am still able to walk without assistance and I am optimistic that this drug will improve my walking even more good luck on your decision
Ed Tobias
Thanks, Mario. Good luck to you, too.
Ed
Joel Vogel
For those reading this blog, please know that the prescribing information we all read for Lemtrada does not include an important area of infection which no one really thinks about because it is not listed: Fungal Infections such as disseminated coccidioidomycosis (Valley Fever for those in the more arid south and southwest) and Histo (for those in the greener wet regions. 4 months to the day of my last infusion I became very ill with 102.9 fevers, sever rigors which made my intention tremors look normal, profuse sweating when I slept to the point of near kidney failure because I would make my bed/pillows/clothes so wet you could wring them out and finally after admission to the hospital with X-Rays, CT Scans, MRIs, Spinal Taps, Pulmonary Lavage and biopsies of the lymph nodes behind my lungs they found I had disseminated coccidioidomycosis and put me on 800 MG of Diflucan, a nasty anti-fungal drug that makes my MS fatigue look like that is running a marathon. I am told I will have it for the rest of my life and will need to take some dose of an anti-fungal until I die or they find a cure for MS because of the suppressed immune system and the fact that Lemtrada just wacked away my T-Cells. All I can say is, I do not think I would do it every again and want all prospective patients to ask lots of questions because of what they are not writing in their clinical trials and shiny brochures.
FROM THE LEMTRADA BROCHURE AND CLINICAL TRIAL SHEET:
"Serious infections: LEMTRADA may cause you to have a serious infection while you receive and after receiving a course of treatment. Serious infections may include:
Herpes viral infections. Some people taking LEMTRADA have an increased chance of getting herpes viral infections. Take any medicines as prescribed by your healthcare provider to reduce your chances of getting these infections.
Tuberculosis. Your healthcare provider should check you for tuberculosis before you receive LEMTRADA.
Hepatitis. People who are at high risk of, or are carriers of, hepatitis B (HBV) or hepatitis C (HCV) may be at risk of irreversible liver damage.
Listeria. People who receive LEMTRADA have an increased chance of getting a bacterial infection called listeria, which can lead to significant complications or death. Avoid foods that may be a source of listeria or make sure foods that may contain listeria are heated well.
These are not all the possible infections that could happen while on LEMTRADA. Call your healthcare provider right away if you have symptoms of a serious infection such as fever or swollen glands. Talk to your healthcare provider before you get vaccinations after receiving LEMTRADA. Certain vaccinations may increase your chances of getting infections.
Oh and by the way, the Lemtrada Central Lab Program for monthly monitoring, at least in Arizona where I can only use LabCorp or EMSI is a joke. They either use the wrong tubes for the blood work, do not draw enough tubes of blood, mishandle the urine sample or just plain loose your sample, delete or misplace your standing lab order and look at you like you have 3 heads when you tell them this is part of the Lemtrada Central Lab Program through Sanofi-Genzyme contracted with EMSI or LabCorp and want you to pay. I was all signed up to be monitored for 4-5 years on a monthly basis, but less than 1 year in I do not know. After nearly a year of fighting with Sanofi-Genzyme, MS-One-To-One, LabCorp, EMSI, my neurologist and I are talking about other alternatives which I would have to pay for to get consistent regular blood and urine monitoring. Needless to say, I have reported these instances to the FDA via MedWatch and telephone calls, so all I can say is check all your options and go in asking every possible question you can think of and those of others.
Hannah
Hi,
I've just been offered Lemtrada after my latest relapse. I felt like my neurologist almost 'sold' it to me as a bit of a wonder drug - but in fairness did explain in detail the potential side effects.
After talking it through with my partner, I think we have decided to put my name on the waiting list (apparently it's quite long!) and then at least it's available to me. I guess I can always change my mind.
I'd just be interested to know about people's experiences? I'm 39 and up until recetly have been doing quite well with the MS - I generally have relapses every 2-4 years depending on how you differentiate a relapse and a flare up. In short, I am pretty much debilatated every couple of years although the length of the symptoms varies.
I've been on Tecfidera for 4 years ish and thought they were doing the job. I'm worried about the psychological effects of the drug as this was something I hadn't really considered. But I'm also just generally worried!
Henrietta
Im sorry for my english, its not my first language. Please let me share my experience from central Europe. I have MS since 1996, I was 15. I was diagnosed 2006. The local neurologist didnt send me to any test. I had about 10 attacks durig that 10 years. I was diagnosed in 2006 and received corticoids, because I didnt meet criteria for any better treatment. I got Copaxone in 2009. Later Tecfidera in 2016. Im positive with JC virus (3,32) Tysabri was not recomended. Our insurance company advised me the Tysabri as dangerous treatment, but not impossible (JC virus is not contraindication for Tysabri, only unsafe). I got Lemtrada finaly in March this year. Infusions were ok, i got also 1000 mg/day corticoids, drugs for fever, for rash, antiviral drugs. I felt good, no pain. I was only sick, couldnt eat much. I ended up with 49 kg, Im 1,75 metre tall. I caught only bronchial pneumonia two weeks later, nothing else. The worst side effect is permanent fatigue, weaknes and trembling. Now its 3 months since Lemtrada treatment and its not better. I feel much worse than before Lemtrada. Doctors say, its not because of Lemtrada, that I feel so bad. And its because im not possitive person and i have always been complaining about weaknes and fatigue. Its true, i have problem with power in my legs and Im very tired quite often. Actually I feel depressed too. I have to go to work, full time and I suffer there. I need to take care of my doughter, who is 6, do all the housework and my body doesnt work. Doctcors are sure, that its impossible that the miracle Lemtrada have such a side effects. I feel like malingerer because I complain. How to get rid of this? Will I feel better for example in half a year? There are only 2 people with Lemtrada in our county. And the other patient feels absolutely fantastic. Is it my fault that I dont? Have I done anything wrong?
Ed Tobias
Hi Henrietta,
Thanks for taking the time to write. Your English is excellent.
I'm sorry that you've been having these problems with Lemtrada. The article that you read is about two years old and I've come a long way since then. I'm now about two months past my Round 2 infusions and doing quite well. I think my cognitive functions have improved (I feel more alert), I'm less tired and I think I'm walking better.
But fatigue was a problem at first. It's not unusual for people to feel very tired for several months after their infusions as their bodies recover. For many of us, six months seems to be the time when things start to improve. My doctor told me "listen to your body." That meant don't try to push too hard or do too much. Rest when you need to rest. Also, drink a lot of water. Staying hydrated is important to feeling better.
You are in a difficult position because you have to work, and do housework and also take care of a young daughter. You have to be Superwoman, but you have a disease that makes you tired and, in addition, you are using a drug that adds to your fatigue. It's not easy and you have a right to complain. However, please try to be positive. And, if it's at all possible, if you have a friend or a family member who can help with housework and your daughter please don't be too proud to ask for their help so that you can get some rest. Tell them I told you to ask. :-).
It's still early in your journey with Lemtrada. Soon, I hope, you'll feel stronger and you may feel some benefit. I've written several times about my experiences. You can find my columns if you search this website for "The MS Wire." Here is a link to my most recent column: http://multiplesclerosisnewstoday.com/2018/04/10/final-ms-disease-modifying-therapy-lemtrada-round-2/
Please let me know how things go for you over the next few weeks.
I wish you well,
Ed
Philip maddocks
Hey people just wanted to advise you all to get food allergy tests done as alot of people with ms are actually allergic to gluten, dairy, legumes and a host of other stuff, check out the american man matt embry who has not had a attack in twenty years, he has a award winning film on itunes that i suggest u all watch it explanis everything, ms is not a mystery it peoples reaction to differnt foods, just by eating mostly heathy, i keep to chicken fish and loads of veg, fruit, nuts and my hands went back to normal within weeks
Susan
My doctor is recommending Lemtrada. I have had an exacerbation every September for 3 hrs in a row. First 2, I had been on avonex for 12 years, last year changed to Copaxone. I haven’t got much better since the last one, last year and now I’m having trouble walking and tingling in my right area. I am so scared of the Lemtrada side effects, well scared is an under statement. I have sent a request to join the FB group for Lemtrada patients, I hope that helps.
Ed Tobias
Hi Susan,
I wrote the Lemtrada column that you read before I started on that med. I'm now almost six months past Round 2. Though the experience has been a bit of a roller coaster ride, overall I'm doing very well and glad that I was treated with the Lemtrada.
Since writing that first column I've written several more about my experiences using Lemtrada. You can find them if you search this website for "Lemtrada Journey." You can also click here to scan through all of my MS Wire columns.
One more thing - There is good information on the Lemtrada Facebook groups. There are two of them and I suggest you join both. However, keep in mind that many more people use these groups to report problems with the med than to report successes, even though the successes outweigh the problems. So, don't be put off by the problem you'll read about. Be aware but not afraid. Above all, however, have a good discussion with your neuro about the risks versus the benefits.
Ed
Kat
Everyone - This is such great information. You have no idea what a relief it is to hear real life stories from people actually taking the drug. I was diagnosed when I was 25 years old. I started Avonex which I had an awful 18 months on while my body adjusted. I've had three or four relapses since, but my neuro told me that I should stick with the Avonex. I had had persistent numbness in both my legs for a couple of years now, so I decided to get another opinion. I managed to score an appointment at the Las Vegas location of the Cleveland Clinic and my new, world renowned neuro says I should have stopped the Avonex years ago given that there were what she considered to be obvious signs that the drug wasn't working. My scans all show that the disease is progressing pretty quickly in me and she recommended Lemtrada. On the one hand: What better time to try the most aggressive treatment than when you are younger and as healthy as you can be given the circumstances? On the other hand: If the drug kills me by way of a brain or liver attack, I will leave my family bearing the knowledge that it was avoidable. Sorry for the morbidity, my wife and I are still trying to decide if we go with Lemtrada.
Ed Tobias
Hi Kat,
I'm sorry you were stuck on Avonex for so long but I'm glad that you finally found a knowledgeable neuro. Avonex was my FIRST disease-modifying treatment back in 1996! Treatments for MS have advanced a lot since then.
You've read a very old column of mine. I'm now seven months post Round 2 of Lemtrada. While it's been a bit of a roller coaster ride of MINOR symptoms over the past two years I think the benefits have outweighed the risks. I've written several columns about my Lemtrada journey and you can find them if you search for "Lemtrada" or "MS Wire" on this web site. There is also a robust Facebook Lemtrada group where you can read a ton of patient experiences. https://www.facebook.com/groups/1723792797845130/
If you go to the Facebook group remember that people with good experiences may not bother to write about them while those with problems tend to vent. From what I've seen, though there can be some severe side-effects they appear to involve only a very small number of Lemtrada patients. And there are monthly lab tests to try to provide an early warning of these possible problems.
Lemtrada seems to be the "knockout" DMT. And, though it's only designed to halt progression, a significant number of its users report a reduction of some symptoms. That's icing on the cake if it happens. It's also said that the younger you are the better chance you have that Lemtrada will do its thing.
So, IMHO, I think that your new neuro is guiding you in the right direction and I hope this helps you with your tough choice. I wish you well. Please let us know what you decide and how things go.
Ed
Vikki
Hi Kat...I agree you are lucky to have found a knowledgeable neuro. I was dx in 1993, at 23 years old. Avonex was the least effective dmt for me in all of the years since then. I did Lemtrada in 2016, second round in 2017. I have had no new progression in my MRI scans although I have had progression in symptoms. I have had no regrets in choosing Lemtrada. Although Lemtrada was not my miracle, I wish it had been around when I was still in the early years. Do your research, it can be a big roller coaster for a few years. The earlier you choose Lemtrada, the better the results will be. I wish you the very best of luck!
Pam
I was diagnosed with MS in 2002. The first and only drug I have ever taken is Lemtrada. I started treatment in phase II of clinical trials. I was randomized to the low dose branch of the study. I believe that is the current dosage now given.
My first infusion was rough. My lack of 'good' veins made them unable to switch IV locations during the week, and by the end of the week of infusions, my arm was on fire. At that time they also were still working on combating side effects. I had a terrible head to to rash, and I wasn't prepared for the side effects from the steroids that I was given along with the infusion of Lemtrada.
The first year of my participation in the study, and I had monthly MRI's and blood draws. The second year, the trial and my infusion was put on hold. I had my second infusion 2 years after the first. It went much more smoothly. They learned how to pre-treat for certain issues, and more importantly, I was able to take 3 weeks off of work for infusion and recovery.
So - the good news. For me, this drug has been miraculous. When I was diagnosed, I had 48 active lesions in my brain, and several in my spine. Since that time - and we are talking 16 years - those lesions are no longer advancing. I have had 1 advancing lesion and one relapse during that time. The relapse was treated with steroids. My Dr. expected me to be in a wheelchair or using a walker in 5 years time when I was diagnosed - before my treatment.
Do I still have MS? Absolutely. Do I know it? Yes. But I would consider it a mild intrusion in my life. I have other health issues that impact me far more greatly.
I did have issues from the second infusion. I went into A-fib 15 minutes before I was to be released from my last infusion. I don't believe it was caused by the drug. It's just as likely from the steroids. It's also something I have had before treatment.
If I were to read about the drug now, there is no way I would take it as the warnings that go along with it are very frightening to me. But I'm glad I was part of the study (and still continue to be - they are still watching us!). Taking the drug was life altering for me.
Ed Tobias
Hi Pam,
Thanks, so much, for all of this detailed info.
As I guess you saw, I wrote this column over two years ago. I'm now nearly 8 months past Round 2 and the benefits have outweighed the problems, which have been slight and few. I've written about my journey several more times. If you're interested you can find them using a search of this website.
It's great to hear from someone who's so far down the Lemtrada road and is doing so well.
And a HUGE thank you for being willing to risk being in an early, clinical trial of a super-charged MS drug for the benefit of all of us.
Ed
Maria
Hey to Everyone. I was diagnosed in 2002 and was on Avonex and did well for 6 years until new symptom of severe pain and the doctor changed me to Rebif. I still have the pain without
Lyrica which treats the nerve pain. I work full time and the pain meds make it very tiring. I have read that this medication can revert some symptoms and I would really like to try it. Does anyone know if the symptom of pain can revert to a little pain and is it possible to work after all the treatments and would I be done with MS drug treatments after I finish round 2?
Ed Tobias
Hi Maria,
I wrote the column that you read over two years ago. I'm now nine months post Round 2 and I'm doing very well. I'm written a lot about my Lemtrada journey. A link to my most recent column is attached and you can search for The MS Wire to find others.
http://multiplesclerosisnewstoday.com/2018/11/30/multiple-sclerosis-lemtrada-slowly-steadily-reversing-ms-symptoms/
Good luck,
Ed
Sara
I’m curious on y’all’s thoughts about using Lemtrada in an effort to hit MS hard and pre-emptively even if you have a mild case of MS. I’m 40 years old, diagnosed 6 years ago. Been on only Copaxone... would love to have the HSCT to completely halt progression. While I’m thankful that my path has been comparatively mild, I’m struggling with the idea of waiting to see how the disease progresses.
Ed Tobias
Hi Sara,
I wrote this column a couple of years ago, before I started being treated with Lemtrada. My experience with this medication has been positive and I've written several of my columns about it. You might also find this column interesting. It's about making the decision you're asking about.
http://multiplesclerosisnewstoday.com/2019/01/04/ms-treatment-decisions-can-cause-gamblers-dilemma/
Ed
MSer
Hi All,
I’m amazed at reading all the comments on Lemtrada. I completed my 2nd round of this Chemotherapy(Lemtrada)drug 8 months ago. I’ve had MS since 2001, I was managing and was active so Neuro said to try this drug to arrest the condition now whilst still good. Little did I know that this would make me even worse.....which it has. 1st round wasn’t good but my neuro said I had to complete the 2nd round 1 year later to see the benefit. Which I did. He is in denial that the chemo has made me worse even though all my family, friends and work colleagues have noticed the decline since the 2nd round. I feel like I’ve been administered this lab monkey/rat drug which has ruined my life, especially as I had recently become a father and feel useless. Strangely the only thing that works for me now is to have a bottle of beer ever couple of weeks and my walking returns to near normal. When I showed my neuro video evidence he went very quiet. I am now just too sceptical about all these drugs and have promised never to take another MS drug again, steroids on the occasion though.
Ed Tobias
I'm very sorry that Lemtrada hasn't worked for you.
I wrote the column about which you're commenting about two and a half years ago. Since then I've completed my Lemtrada treatments and I'm now 1 year post Round 2. My disease progression seems to have stabilized and a few of my symptoms (gait, urinary and bowel) seem to have improved a bit.
I've been fortunate. Lemtrada has worked for me, as it appears to work for many people treated with it. But it doesn't work for everyone. I don't think any MS medication does.
Ed
Sonya Gatica
I was diagnosed with MS in 1993. I went from Avonex to Tysabri. I was on Tysabri approximately 10 years. I started Tysabri as a study patient. My JC Virus was climbing and climbing, my neurologist switched me from an every 4 weeks infusion to every 6 weeks. Then my JC Virus started going back up again. I asked my neurologist about Lemtrada and he did not see a reason why I wouldn’t do it. My first dosage was in August 2017 and my second September 2018. I would have to say I was scared and had some ups and downs but over all I do NOT regret doing the treatment. 2 years out from my 1st infusion and I’m doing great!!!!
Ed Tobias
Hi Sonya,
I'm glad to hear you're doing so well. The column on which you're commenting was written several years ago, before I began Lemtrada treatments. I'm now 15 months post Round 2 and am doing very well. I'm written several columns about my journey over the past 3 years. You can find them if you search for "The MS Wire" and then either scroll through all of my columns or search the columns for "Lemtrada."
Good luck,
Ed
cynthia
It makes me feel optimistic that others have had such good results from Lemtrada. Unfortunately, I have not. I keep waiting for the positives to occur.
I had my first infusion at the end of June 2019 (now October) and I am anxiously waiting for things to turn around.
I have hand coxsackie virus (hand foot and mouth) and herpes zoster (shingles) since then. The coxsackie although no longer active, has caused my tongue to stay sore and sensitive. With my zoster, the rash has dissipated but am now dealing with post herpetic neuralgia.
My spasms in my legs are SEVERE, although I am not necessarily blaming this on Lemtrada. I am taking tizanidine at bedtime (cannot during the day because it makes me or puts me in a stupor and drowsy) and baclofen during the day.
I also am on Magnesium and Verapamil for my spasms.
When I am not having spasms, my legs ache.
Weakness, cog/brain fog and balance, are my worst symptoms. I do not have surface sensation in areas and very minimal in others. I am a physician and continue to work so I take Modafanil for focus.
I do not have any other health issues. My blood pressure has always been on the lower end of normal (100/50), no heart disease or diabetes, low cholesterol and am in the middle of my healthy weight range (bmi 21.8%) and follow a gluten free diet.
So I guess my question is that how long after the infusion did many of you start to improve? I am struggling majorly. I am trying to stay in the workforce because I am not the type to give up. Some days, many days, are bad for me. I actually currently am having more bad than good,
Would appreciate any insight or suggestions you all may have.
Ed Tobias
Hi Cynthia,
I'm sorry that you're having all of those problems but four months isn't very far into your Lemtrada journey, so good things can still happen.
The column on which you're commenting is over three years old and was written before I began my Lemtrada treatment. I've written several columns since then about my treatment. You can search and read them but the bottom line is this: For me the first six months after my first treatment were a roller coaster ride of poor energy and symptoms that seemed to improve and then got worse. But now, 18 months after Round 2, my disease is stable and some symptoms - bladder, bowel, fatigue, walking - have improved a little.
Remember, Lemtrada is only supposed to slow or stop disease progression. Any symptom improvement is just icing on the cake...you shouldn't expect it.
Good luck,
Ed
Jonathan Sturgess
I have now finished my 2nd stint of infusions and do feel a little bit better in my self ,at least I don’t think I’m going to be in that wheelchair in 5 yrs as I was told if I didn’t have treatment , but I am now hearing that Lemtrada has been shelved here in the UK but it has done nothing but giving me back my life .
Ed Tobias
Hi Jonathan,
The column that you've responding to it a few years old. I'm now 18 months post Round 2 and I'm doing well. No side effects now and a little improvement in some symptoms.
The UK has temporarily halted approval of Lemtrada as a first-line treatment, except in unusual circumstances. That's a shame because I'm a believer in "hit it fast, hit it hard" when it comes to treating MS.
Best of luck to you,
Ed