Stopping Fingolimod – Gilenya – for Pregnancy Raises Relapse Risk

Women with relapsing-remitting multiple sclerosis (MS) who suspend their use of fingolimod — sold as Gilenya, among others — to conceive or during the early stages of pregnancy have a significantly higher risk of relapse during and after pregnancy, a new study finds.
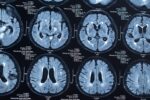
Stopping fingolimod resulted in a greater proportion of women developing new or enlarging lesions, as well as lesions with active inflammation, the results showed. In fact, 22% of the participants experienced relapses during pregnancy, while 44% of the women had them afterward, according to the researchers.
“In our study, we observed significant disease reactivation during pregnancy (mostly in the first trimester) and after delivery,” the researchers wrote.
“Women who discontinue [fingolimod] to become pregnant need to be closely monitored given the risk of disease reactivation,” they added.
The study, “Disease Reactivation after Fingolimod Discontinuation in Pregnant Multiple Sclerosis Patients,” was published in the journal Neurotherapeutics.
MS is most common in women of reproductive age, and evidence shows that many disease-modifying therapies or DMTs are harmful to women who want to conceive, are pregnant, or are breastfeeding. However, stopping DMTs such as fingolimod — marketed as Gilenya and as generics — or Tysabri (natalizumab) can cause severe relapses.
Scientists, to date, have limited knowledge about how frequently relapses occur in women who suspend fingolimod treatment due to planned or unplanned pregnancies. They also are unsure of the factors that predict relapses once fingolimod treatment is stopped.
Understanding these factors could help in counseling women with MS who intend to become pregnant or have an unplanned pregnancy.
Now, to learn more, a team of researchers in Italy investigated the frequency and predictors of relapses in women with relapsing-remitting MS who stopped fingolimod to plan a pregnancy or upon finding out they were pregnant. The study took place at four Italian centers between 2013 and 2019.
A total of 27 women with a median age of 29 were included in the study. They had been diagnosed with MS a median of 9.1 years before stopping fingolimod, and had used the medication for a median of 2.9 years.
Before fingolimod, these patients had experienced a mean of 1.3 relapses per year, but this measure — called an annualized relapse rate (ARR) — dropped to 0.2 during fingolimod treatment. Of note, ARR was only 0.04 during the last year of treatment, with just one woman experiencing a relapse.
During pregnancy, however, six women experienced a total of 10 relapses. The mean ARR during pregnancy (0.49) was significantly higher than in the last year before pregnancy.
Most relapses occurred in the first trimester, with four women experiencing six relapses. Yet, relapse rates throughout pregnancy remained significantly lower than the ARR before fingolimod’s start.
In the first year after delivery, 14 women experienced a total of 18 relapses, and all six women who relapsed during pregnancy had at least one relapse in that period. Similar to the pregnancy period, the mean ARR in the first year after delivery (0.67) was also significantly greater than that reported in the last year before pregnancy.
In the combined period of pregnancy and first year after delivery, the mean ARR was 0.62, which was also significantly higher than the mean ARR reported in the last year before pregnancy. However, it was considerably lower than the mean ARR reported before the women started fingolimod treatment.
A couple of months after delivery, more women (63% vs. 30%) showed new or enlarging brain lesions on brain MRIs as compared with their radiological assessments before pregnancy. Gadolinium-enhancing lesions, which are those with ongoing inflammation, also were more frequent (44% vs. 0%). Both lesion types were found more often in patients who relapsed after discontinuing fingolimod.
According to the researchers, pregnancy planning potentially exposes women to an extended period without DMTs, which increases relapse risk. In this group, 11 women had planned their pregnancies and stopped using the therapy for a mean of 88 days, while the remaining 16 had unplanned pregnancies. Interestingly, there were no differences across both groups in terms of relapse rates or brain lesions.
Of the 27 women who became pregnant, 26 gave birth after a mean of 38.2 weeks. Of note, a normal pregnancy is about 280 days or 40 weeks, though babies are considered full term after 37 weeks. One planned pregnancy resulted in a miscarriage at 10 weeks.
While 16 pregnancies were unplanned and the unborn babies were inadvertently exposed to fingolimod, all of the fetuses developed normally with no deaths. All planned pregnancies save one also resulted in healthy, live fetuses.
After delivery, 21 women resumed fingolimod while six switched to other treatments. The women resumed treatment after a mean duration of 132 days, or about 4.5 months.
Factors like age, body mass index, disease duration, disability scores, ARR before fingolimod treatment and before pregnancy, or MRI activity did not significantly associate with relapses during pregnancy. However, women who experienced relapses during pregnancy were significantly more likely to relapse after delivery.
The researchers noted some limitations, including the small sample size and the retrospective design. Still, the study provides valuable information to guide women taking fingolimod who plan a pregnancy or become pregnant while using the drug.
“Clinicians should alert women with MS on [fingolimod] treatment who are planning to conceive about the possibility of increased disease activity after [fingolimod] discontinuation, even if adequate prolonged control of disease activity [has been] achieved with therapy,” the researchers wrote.
Furthermore, women who relapse during pregnancy should also consider resuming highly effective disease-modifying therapies soon after delivery to lower the risk of future relapses, the researchers said.