FAQs about the PoNS device
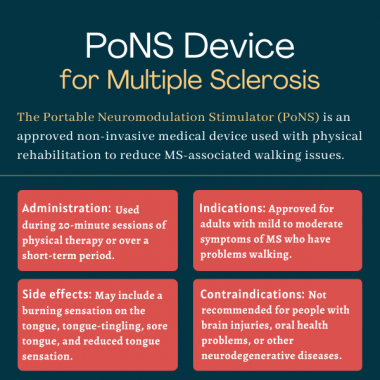
The U.S. Food and Drug Administration approved the PoNS device in March 2021 as a short-term treatment for walking problems due to mild to moderate symptoms of multiple sclerosis. The approval is for use in combination with supervised therapeutic exercise programs in adults ages 22 and older.
Clinical trials investigating the PoNS device have not accepted people who were pregnant, so it is unclear whether the therapy is safe in such conditions.
No study has assessed the effects of alcohol on PoNS use, or vice versa, but alcohol can interfere with brain activity, some medications, and disease symptoms. Therefore, patients should discuss this topic with their healthcare provider.
Some patients in clinical trials have seen improvements in walking ability within the first two months of use. But the intensity of PoNS electrical stimulation can vary from person to person, and the targeted exercises are tailored to a person’s individual needs. Thus, there can be a lot of variability in the time it takes for PoNS and the exercise intervention to produce evident benefits. The earliest time point reported for improvements in walking skills in MS clinical trials was six weeks.
Hair loss and weight gain were not reported as side effects of PoNS in clinical trials. Patients should talk with their healthcare team if such events occur.
Related Articles

 Fact-checked by
Fact-checked by