Adult myelin-making cells display unique marker affecting activity
Differences seen in neonatal and adult cells may aid remyelination therapies
Written by |
A specific epigenetic marker, or a chemical modification in DNA that alters gene activity, may explain why adult oligodendrocyte progenitor cells respond differently to therapies aiming to restore myelin than their neonatal counterparts, a study reports.
The modification, called a lysine eight acetylation on histone H4, helps to regulate the activity of these myelin-making cells in the adult brain.
This finding “holds promise for developing more effective therapies for conditions characterized by myelin damage like multiple sclerosis, Alzheimer’s, and several psychiatric disorders,” Patrizia Casaccia, MD, PhD, the study’s principal investigator and director of the neuroscience initiative at the City University of New York (CUNY), said in a university news release.
The study, “Histone H4 acetylation differentially modulates proliferation in adult oligodendrocyte progenitors,” was published in the Journal of Cell Biology.
Inflammation in MS damages the myelin sheath and nerve cell signaling
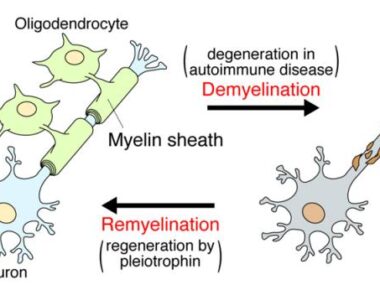
Multiple sclerosis (MS) is due to inflammation in the brain that damages the myelin sheath, a fatty covering that wraps around nerve fibers and helps them send electrical signals, a bit like rubber insulation around a metal wire. Damage to myelin disrupts nerve signaling, ultimately driving disease symptoms.
One of the main goals of modern MS research is finding treatments that can help repair or replace damaged myelin.
Within the brain, myelin is made primarily by cells called oligodendrocytes. When myelin is damaged, immature cells called oligodendrocyte progenitor cells (OPCs) can spring into action, growing into mature oligodendrocytes to repair myelin. This remyelination process is impaired in MS, though the reasons aren’t fully clear.
In addition to helping repair damaged myelin in adults, oligodendrocytes and OPCs are essential for making myelin during neonatal development. Both neonatal and adult OPCs share many characteristics, but they also have notable differences in their biological activity.
Researchers with CUNY conducted a battery of molecular experiments aiming to understand reasons for these differences.
“By focusing on adult OPCs, we can move closer to repairing myelin damage and improving patient outcomes,” Casaccia said.
Specifically, they focused on epigenetic changes within the cells. Epigenetics refers to chemical modifications to DNA molecules that don’t alter the genetic code itself, but influence how specific genes are turned on and off, substantially impacting the activity of cells.
Results showed that, compared with their neonatal counterparts, adult OPCs carry substantially higher levels of an epigenetic modification called lysine eight acetylation on histone H4.
Histones are proteins that help to keep DNA molecules in place; strands of DNA wrap around histones sort of like thread wraps around a spool. In this modification, a particular type of histone — H4 — undergoes a chemical modification called acetylation at a particular site, known as lysine eight.
“The identification of this histone tag provides a clearer understanding of OPC proliferation in the adult brain,” Casaccia said.
This modification was particularly abundant close to several genes that are known to be important for the activity of OPCs, and the researchers demonstrated that this modification is essential for regulating the activity of adult OPCs.
Importance put on targeting adult-specific cell mechanisms in research
When the cells were treated with chemicals to block the modification, their ability to proliferate and grow into more OPCs was impaired. The same chemical treatment didn’t substantially alter the growth of neonatal OPCs, lending further credence to the idea that this modification is specific to adult cells.
“These data identify [lysine eight acetylation on histone H4] as important for the identity and proliferation of adult oligodendrocyte precursor cells,” the researchers concluded.
Findings also “underscore the importance of targeting adult-specific cellular mechanisms in neurotherapeutic research,” said David K. Dansu, PhD, a former doctoral student at CUNY working as a postdoctoral fellow at Regeneron Pharmaceuticals.
Researchers now plan to explore the effects of this modification on cellular activity and its implications for potential treatment strategies to promote myelin repair.
“Our future investigations will aim to further elucidate the role of lysine 8 acetylation on histone H4 and explore its potential in clinical applications,” said Ipek Selcen, a doctoral student at CUNY.