Test-Walking the New Bioness L300 Go


I’ve been using a Bioness L300 for just over five years to counter my foot drop. Without the L300 strapped to my left leg, it’s difficult for me to walk more than 25 or 30 steps, even with two canes.
The L300 is a functional electronic stimulator (FES). Each time I start to lift my left leg to walk, it sends a low-intensity electrical pulse down a nerve that runs from my knee to my ankle. That pulse forces my foot to flex upward from my ankle so that my toes don’t drag. The electrical pulse replaces the signal that my brain should be sending to the nerve that my MS has blocked, and it counters my foot drop.
About six months ago, L300 manufacturer Bioness received the OK from the U.S. Food and Drug Administration to market a new model of the L300, the L300 Go. A few days ago, I had an opportunity to “test-walk” the Go, and here’s what I found.
No foot sensor needed
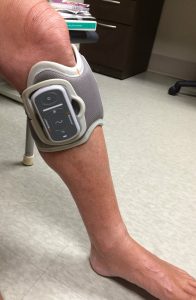
(Photo by Ed Tobias)
The biggest and best difference is that the L300 Go doesn’t require you to use a foot sensor in the heel of your shoe. In the original L300, the sensor is used to detect your motion as you begin to walk. It sends a Bluetooth-like signal to the cuff on your leg, telling it that you’re trying to move. The cuff then generates the pulse that stimulates the nerve in your leg and helps you to lift your foot.
That means that anytime you change shoes, you need to move the sensor to the pair that you’re going to wear. Not only is that a nuisance, there can be a lack of consistency in where you place the sensor in the shoe. Also, because insoles are different, there can be the same inconsistency in the amount of heel pressure that’s required to trigger and to end the pulse.
Naturally, you can’t use the original L300 if you’re barefoot.
3-D motion detection
Without a foot sensor, the L300 Go’s cuff detects the start of your leg motion. This is similar to the process used by the WalkAide, a competitor of the L300. Unlike the WalkAide, the L300 Go has a 3-D-motion-detection system. Not only does it detect the forward and backward motion of your leg, it also detects sideways movement and leg rotation. This allows a physical therapist to adjust the unit more precisely to make it more responsive to each patient’s unique gait. To provide this three-way detection the Go contains four electrodes, compared with the original unit’s two.
No control unit
The original L300 requires you to carry a small control unit, which you use to turn the device on and off, put it into “test mode,” and also to adjust the intensity of its pulse. With the Go, this is all done on the side of the cuff. (If you really want a foot sensor or a control unit, Bioness will sell you one. But why would you want one?)
The test walk
The Go looks and feels like the original L300. Maybe I walked a little faster with it. Maybe it triggered a little quicker. I don’t think that it allowed me to walk any further than normal. On the other hand, it was certainly nice to be able to sit in a chair without having to turn off the unit so that it wouldn’t trigger when I released the pressure on a heel sensor. Unfortunately, I forgot to test the Go walking up and down stairs. My guess, however, is that it would work better than the original unit because its trigger mechanism isn’t dependent upon heel pressure.
What’s it going to cost?
The Bioness rep told me that the Go will cost the same as the current L300. That’s about $6,200. I was also told that there would be a discount if current users wanted to upgrade, but she couldn’t say how much it would be.
Don’t count on insurance picking up the cost. Bioness apparently has had greater success recently getting insurance to pay for the L300 than when I got mine in the fall of 2012. But, it’s a fight and a lot of really good documentation is necessary. The same goes for Medicare and Medicaid. Again, don’t hold your breath waiting.
Do I plan to upgrade?
Nope, not at that price. Even with a discount, the benefit that I’d receive probably wouldn’t be enough to justify the cost of the upgrade, at least not now.
Bioness tells me that it intends to support the original L300 units for “at least the next three years.” So, I guess if my unit breaks down in 2020 or later, I may have to go for the Go.
If the L300 Go is right for you, however, Bioness expects to begin shipping it in October to patients whose doctors have given them a written order.
You’re invited to follow my personal blog at: www.themswire.com.
***
Note: Multiple Sclerosis News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The opinions expressed in this column are not those of Multiple Sclerosis News Today, or its parent company, Bionews Services, and are intended to spark discussion about issues pertaining to multiple sclerosis.







Michael Davoult
That is very interesting reading about this "new" model. I received my device in January of this year and wish I would have waited for the new design. My insurance paid for around 80% of the cost for the old design of Bioness Elect Stimulator.
Tom Carson
Aloha,
Thanks for your post. I am concerned about insurance coverage and want to learn about other people's experiences to approach it properly. Can you share your experience? what insurance do you have, did you get a referral from your PCP or did you need to get the prescription? things like that.
Mahalo nui loa,
Cyndi
Great article - thanks! Do you know if Bioness has a "try before you buy" program? I would definitely make the investment if a trial period proved it works for me. Thanks again for your insights.
Ed Tobias
Hi Cyndi,
Thanks for your comment. The Bioness rep told me that they have a "rent before you buy" program, which will let you rent one for a month. If you decide to buy the month's rent is applied to the purchase price. I don't know what the cost of that month's rent will be.
Good luck,
Ed
Cyndi
Awesome! Thanks Ed, I will check it out. Cyndi
kevin
is the l300 in Australia yet
Kezza
It is in Australia now as of November 2017. It's almost $10,000au
Amy
I was able to test the Bioness L300 a few years ago at my orthodist's office. At that time the rep mentioned that they were coming out with a new and improved one. When I saw the video for the pro, I was excited! I haven't tested the pro yet, but even the old one was a frickin game changer! I haven't been able to figure out a way to afford the device yet, but I am working on it. I am not a fan of my AFOs. I would have a lot more freedom with a Bioness.
kevin
is the l300 in Australia yet
Linda Hunter
Yes it is Kevin
John
I would like to to rent to see if I could walk. Please send me info asap.
Ed Tobias
Hello John,
I am a writer, not a representative of this company. I suggest you look at their web site, www.bioness.com, and contact them directly. But first you should speak with your neurologist about this, since you'll need a doctor's order.
Ed
Barton
I'm a current user of L300 devices for both legs. I was just told by Bioness that the electrodes (which need to be replaced every two weeks) will only be available until 2020. After that, I guess I'll have to buy the new models and be stuck with two expensive paperweights.
Habeebah
I found the representives very rude. I've had my device for two years and voiced my disappointment in the trade in. I paid cash for my device and with the trade in I would be spending close to 18k. Didn't not like the email the phx rep sent me.
Habeebah Abboushi
Ed Tobias
Hi Barton,
After reading your comment I contacted Bioness to ask about this. The company tells me that the electrodes that are used with the L300 Go can also be used with the L300. So, you (and I) shouldn't be left holding a paperweight.
I was also given the impression that a different device that Bioness makes, and that had an upgrade back in 2011, is still being supported. So, that 2020 "unsupport" date for the L300 may not be as firm as some of the marketing reps would like you to think it is.
Barton
Thanks, Ed!
I hope that's true. I use the large cloth electrodes that have 2 snaps or connection points. It looks like the new devices have 3 snaps. Maybe they're talking about the smaller circular electrodes or the hydrogel electrodes?
I've been trying to find out what the upgrade cost but I haven't been able to get an answer so far. If anyone here has heard anything about the upgrade cost, please share!
Ed Tobias
Barton,
I assumed that you were referring to the gel electrodes, which are what I use, so that's what I asked about. The L300 Go actually has 4 electrode contact points which, apparently, are necessary to achieve the 4-way (rather than 2-way) adjustment for the Go. So, I'd have to guess that the cloth electrodes for the Go won't work on the original. However, I'll see if I can confirm that.
As for the cost, as I wrote I was told the cost of the Go will, at least right now, will be the same as the price of the old unit, with some percentage discount for current users. I don't know more than that, so I'd suggest that you ask Bioness Customer Service directly.
Barton
Ed:
I have asked Bioness Customer Service directly about the percentage discount for current users. But they told me I need to talk to a particular Client Management Associate to get the answer and that person has yet to return my call from a week ago. The same person's voicemail says for immediate assistance please contact my associate (names left out intentionally) and that person also hasn't returned my call. Which is why I hoping someone around here had heard about the percentage discount for existing users. I guess I have until some time in 2020 to get the answer. But not dealing with the foot sensor is a pretty appealing prospect.
Jean
I'm also looking into the Bioness Go. I have had the L300 for 6 months and was interested in the deep discount they were offering. I have spent 2 weeks e-mailing and leaving messages on the phone(no one will return my call). I have reached other people but it seems only one woman can answer questions. She did e-mail today and said I could trade in my device and pay $4500 for the new one. How fair is this? My L300 is only 6 months old, I would expect more of an even exchange, it was hard enough to get the funds the first time . Not sure how I can do it again. Insurance pays nothing and my medical bills are high enough and now it seems that my device willnot be supported much longer. Not sure how many people will be able to afford this. I cannot even get an answer as to how much it actually costs. I have found the Rep to be extremely rude and am losing faith in this company.
Ed Tobias
This is very disappointing, Jean. Thanks for sharing.
Barton
Thanks, Jean. I guess this explains why Bioness has been so reluctant about divulging the price for existing L300 users. Between phasing out support and the necessary supplies for existing L300 users and the price for the upgrade, it certainly puts the company in a pretty bad light.
Kevin Lipetzky
I have just started thinking about a FES. Just like car shopping. How many makes & models are available? Perhaps, a review comparing models, price, warranties, insurance coverage ect. I don,t know where to begin. Please advise.
Ed Tobias
Hi Kevin,
I think the only two functional electronic stimulators on the market for foot drop are the Walk Aid and the Bioness L300 Go. The original L300 is no longer being produced. Bioness also makes an FES that's designed to stimulate the nerve that controls the hip flexor.
Both companies have web sites that can give you more info. I don't know the price of the Walk Aid but I think the L300 Go runs about $6,000. It's very difficult to get insurance or Medicare to pay for any of that cost. I don't know about healthcare systems outside the US.
An FES needs to be prescribed by a doctor and fitted by a physical therapist. Speaking with one of your medical professionals should be useful. Also, call Bioness customer service. They have an 800 number and might be willing to set up a trial. I don't know about Walk Aid.
Ed
Marek Grabowski
I have just scheduled appointments for both WalkAide and Bioness! My insurance verbally claims that it would cover either of the devices 100%! I am in Denver, CO.
Thank you for bringing these to my attention.
Ed Tobias
Hi Marek,
Sorry for the delay in approving your comment. I was away for a couple of days. It's great that you're checking out both devices and I hope that one will be right for you. It's even better that your insurance company says it will pay for it. Please keep us posted on how they impact your walking and if your insurance really does pay for it.
Ed
Mack
For similar treatment you may want to try a product from XFT (a Chinese company). The G3 model is sleeker and cost much less (~$1600). Hope this helps.
kim thomas
i have not used this device yet but very interested.
i am currently using an AFO from Canada, the one that fits outside the shoe. and i found that out - don’t buy any device directly- go to an orthopedic that’s fits AFOs, etc, and let them order the device and for it, so if any problems, they will take care of and you won’t have to deal with the manufacturers. and get a doctor’s prescription
Ed Tobias
You're absolutely correct, Kim. It's best for an AFO to be fitted to the person who will be using it. It's not really one-size-fits-all. especially if it's being used to correct foot-drop. A specialist in orthotics or prosthetics should do the fitting and, as you say, a prescription would be needed.
Ed
Barton
I tried for nearly a month to get the upgrade price information. And never received a return call. That was nearly a year ago. Just today, I received an email saying "the L300 Go upgrade program is set to expire on October 31st. Act now to take advantage of this offer. Upgrading is as easy as 1, 2, 3." So I decided to give it another shot and call. I guess I'm a glutton for punishment. Of course, I couldn't get through to a rep. Maybe I'll get an answer before 2020.
Ed Tobias
Hi Barton,
That's very discouraging news and it's a heck of a way to run a business. Would you be willing to forward the Bioness promotional email to me so that I can try to find out what the problem is? ([email protected])
Thanks,
Ed
Barton
Email sent. And I actually got a call back from Bioness today. I included what I found out about the upgrade cost and inability of using the new electrodes with the old models. Thanks, Ed!
Mark
I've noticed that the L300 Go is now available through the prosthetic company Ottobock. They have a good reputation in Australia and might be able to offer a free trial.
Ed Tobias
Thanks for that info, Mark.
Here in the US I was able to try my old L300, and later the L300 Go, but only with a company physical therapist in a clinic. No "take home" trial. It would be nice if that would be offered in Australia because it's hard to just whether this expensive device is useful without trying it in a real world setting.
Ed
Barton
I bit the bullet and paid for the upgrade from the L300 to the L300 Go. It made me ill to pay so much for the upgrade after buying the L300s just 2 years ago.
I don't notice any significant improvement in my walking over the old model. But the fact that the new model doesn't require a shoe sensor is a massive benefit for me. It was always a long process switching the sensor from one pair of shoes to another. And it's great to just wear around my home without shoes. The iPhone control app is a nice addition too. It's a little finicky but it beats carrying around the remote. So thumbs up from me on the L300 Go. Not so much for the upgrade price and policy.
Ed Tobias
Hi Barton,
I hear you.
As you know from reading my column, I also didn't see a great improvement in my walking when using the new model. But I agree that I'd love to be rid of sensor. Sooner or later I may have to go for the Go, but I'm not rushing to do it.
Ed
Jim
I have recently been diagnosed with MS at the age of 53. I need to be on a tight budget but my neurologist insists on me aquiring th L300/300Go. Insurance will not cover cost. Can someone direct me towards either a copay assistance program or a financial assistance program?
Ed Tobias
Hi Jim,
When I first purchased my L300 Bioness offered me one that was used as a demo. I don't know if they still do that, but it couldn't hurt to check with their sales people.
The Multiple Sclerosis Association of America offers some assistance for some assistive equipment. I don't know if an item as expensive as the L300 Go would be on their list, but I'd suggest you look into that source. https://mymsaa.org/msaa-help/assistive-equipment/
Good luck,
Ed
Karon Sutton
Loved all your comments.
Ed Tobias
Thanks, Karon.
I wrote that column about a year and a half ago and I'm going to give the L300 Go another try at the end of this month. So, stay tuned.
Ed
Madeline grosso
Please. Have someone contact me to try ty
Richard Vega
I’m in the process of getting my PC, Neurologist, and my pain management doctors to get the “pre-determination “ request into my Insurance company so hopefully they will approve the device for my drop foot. I’ll let you all know how it goes. Wish me luck.
Ed Tobias
Hi Richard,.
Unfortunately, you're going to need LOTS of luck. I finally bit the bullet and have upgraded to the L300 Go. When I did that I asked the Bioness people if anything had changed regarding insurance paying for the device. I was told that very occasionally they're able to get approval. But Medicare continues to deny it for use by people with MS and most insurance companies follow the Medicare guidelines.
I have an appointment, tomorrow, with a Bioness PT to fit and adjust the Go. After I've used it for a while I'll be writing another column about my experience with it.
Ed
Lee Thompson
Are you only looking at this one. I'm going to test one from Hangar in about 2 weeks. The first test went well. Is there a place to compare others or you just with this company?
Ed Tobias
Hi Lee,
I wrote this column about two years ago, when Biogen first introduced the L300 Go. As someone who, at that time, had used the L300 for about five years I wanted to try the newer model. At the time I wasn't impressed. However, I've finally broken down and switched to the "Go." I now have an improved opinion of the "Go" and have traded in my original model for the new one. I'll be writing a column about my experience right after Thanksgiving.
By Hangar I assume you mean the Walk Aide FES, which is sold on the Hangar website. As I think I mentioned in my column, I tried the Walk Aid but I didn't like it as well as the Bioness. As far as I know, the Bioness L300 "Go" and the Walk Aid are the only units of this type that are available.
Ed
Wendy
Hi Ed; read all the posts and your answers with interest; do you have an update to share since last one posted November 25, 2019?
Ed Tobias
Hi Wendy,
I wrote a new column on December 3, 2019 with a full update. The bullet points are: It helps me, it's better than the original L300 and (as I'm sure you realize) it's expensive. Here's a link to that column: http://multiplesclerosisnewstoday.com/columns/2019/12/03/a-bioness-l300-go-is-now-on-my-leg/
I hope this info helps you,
Ed
James
Ed, I just found out about this device yesterday 7 Feb 2022 after my neurology appointment at the St. Louis VA. It sounds as though they are going to outfit me with one and I couldn't be more excited. Have you noticed any difference in your distance you can traverse with it on? I get exhausted fairly quickly now and just want to regain some semblance of normalcy. Thanks!
Ed Tobias
Hi James,
I've glad you found out about the Bioness and very glad the VA will pay for it. The VA is the only organization that I know of that will pick up the cost, and it's not cheap.
When I got my original unit, about 10 years ago, it noticeably increased my walking distance. It even gave me the ability to walk up a short, grassy hill. That would have been impossible without it. If you search for "L300" on this website you should see some of my earlier columns about this unit. They'll give you an idea of the ups and downs.
Good luck,
Ed
Thomas Boylan
I reside outside the US and have been attempting to contact the company the manufacture/supplies the Bioness 300Go so I can find a rep in my country
Would anyone have the contact information, phone or email.
Ed Tobias
Hi Thomas,
Here's an email address:
[email protected]
There's also a contact form provided on the company's website:
https://www.bionessmobility.com/dtcinquiries
Hope this helps,
Ed
Maria Dulisse
Hello
My 38 yr old daughter has MS and has difficulty walking and has to use a walker. She doesn’t have foot drop. Do you think the Bioness L300 GO will assist her with her gait so that she does not need the walker?
Ed Tobias
Hi Maria,
I'm not a medical professional, just a person living with MS. However, the L300 GO is designed to flex the foot upward from the ankle as the person walks. It's very unlikely that she would be able to discard her walker just because she was using the L300 GO. I need to use two canes with mine and wouldn't be able to walk without it. But, I would ask her neurologist or physical therapist what he or she thinks.
Ed
Jay Visnjic
I read all posts available on this site. Everyone is talking about challenges purchasing or upgrading L300 GO. Can someone tell me if this gadget actually helps with walking? I am thinking about purchasing one for my wife.
Greatly appreciated
Jay V
Ed Tobias
Hi Jay,
The answer is sometimes it helps. It helped most when I first got it, several years ago. It allowed me to walk up a grassy hill using just one cane, something I couldn't do without it. But my MS has progressed since then and it gives the Bioness more of a challenge. The L300Go's cuff also has to be placed fairly precisely on the leg so that its effectiveness varies depending on how accurate my placement is and how clean (free of any soap residue or oil) my leg is. Even so, I went to a restaurant the other night without the scooter that I usually have in the car and had to walk about a block from where I parked. I couldn't have done that without the L300.
Bioness used to have a program that allowed you to rent a unit for a month with an option to buy it, so that someone could try it out. You might give their customer service number a call and ask about it.
Ed
Stephanie L. Benkendorf
How can I test this device to see if it will be of help to me?