FAQs about the myelin sheath and MS
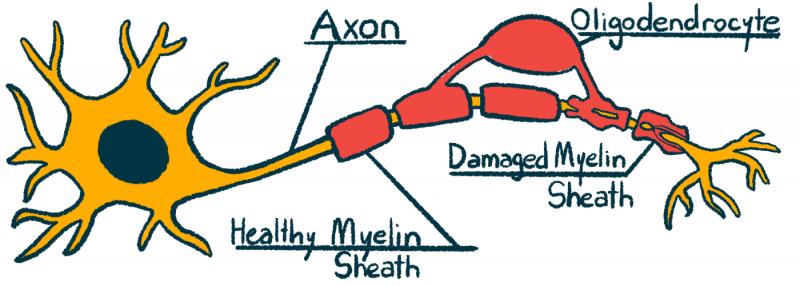
Multiple sclerosis is caused by inflammation in the central nervous system, comprised of the brain, spinal cord, and optic nerves. This inflammation causes damage to nerve cells and results in demyelination — the destruction and loss of the myelin sheath, the protective coating around nerve fibers. Such damage to the central nervous system mainly is responsible for the symptoms of the disease.
Myelin damage in people with multiple sclerosis can be detected by MRI scans. On these imaging scans, areas of myelin damage and inflammation appear as lesions. There are several types of advanced MRI techniques and analytical strategies that can be used to assess damage to the myelin sheath. These include: magnetization transfer (based on the use of radiofrequency pulses to estimate myelin content); myelin water fraction imaging (based on the production of myelin water maps — by looking at water molecules “trapped” between the layers of myelin sheath — to assess myelin status/content); and diffusion tensor imaging (a technique to estimate myelin content based on tissue structure information obtained by measuring water diffusion).
The myelin sheath is needed for nerve cells to efficiently send electrical signals. If myelin is damaged or destroyed, the normal flow of these signals is interrupted and may lead to symptoms. The specific symptoms arising from damaged myelin depend on where the damage has taken place. For example, damage to the optic nerves (that connect the eyeballs to the brain) may result in visual symptoms, whereas damage in nerve cells of the spinal cord is commonly linked with motor symptoms, sensory problems, and trouble controlling the bladder and bowels.
It is possible for the body to repair a damaged myelin sheath. This process is led mainly by myelin-producing cells called oligodendrocytes. However, myelin repair is not a very efficient process in patients with multiple sclerosis. Thus, finding ways to promote better myelin repair is an active area of research.
B12 is a vitamin that is necessary for maintaining the health of the myelin sheath. Abnormally low B12 levels may cause MS-like symptoms. However, there is no robust evidence to support that B12 supplements provide additional benefit to patients with multiple sclerosis who have normal levels of the vitamin.
Related Articles

 Fact-checked by
Fact-checked by