FAQs about ibudilast in MS
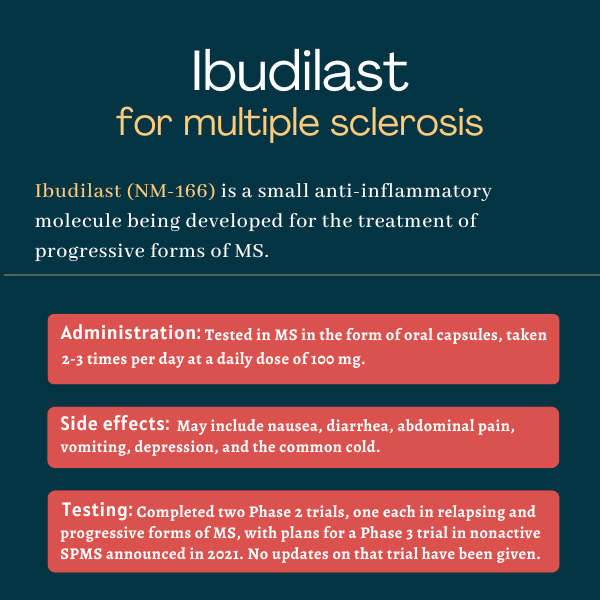
Ibudilast is an experimental therapy designed to reduce inflammation and neurodegeneration in multiple sclerosis (MS) by modulating the levels of several immune signaling molecules. In clinical trials, ibudilast significantly slowed brain volume loss and reduced disability progression in certain people with MS.
Ibudilast has shown encouraging results in Phase 2 clinical trials, but further Phase 3 studies are still needed to confirm the therapy’s safety and efficacy in multiple sclerosis patients. Even with its fast track designation from the U.S. Food and Drug Administration, which is meant to accelerate its clinical development and regulatory review, it may take several years before the medication can be submitted for regulatory approval. Therefore, there is no specific timeline for when the FDA might approve ibudilast.
Clinical trials of ibudilast have not included people who were pregnant, and all participants who had the ability to reproduce were required to use effective methods of contraception while on treatment, and for one month after their last dose. It is not known if the therapy is safe during pregnancy.
Multiple sclerosis is different in every patient, and responses to treatment also will vary. Still, in multiple sclerosis clinical trials, a significant reduction in the rate of brain atrophy (shrinkage) was evident after one year of ibudilast treatment in both relapsing and progressive forms of the disease.
Neither hair loss nor weight gain have been reported in multiple sclerosis clinical trials as side effects of ibudilast. Patients should talk with their healthcare team if such events occur.
Related Articles

 Fact-checked by
Fact-checked by