Experimental Relapsing-Remitting MS Drug Treatment Advancing
Written by |
Synthetic Biologics, which specializes in the development of therapies for pathogen-specific diseases, recently announced the publication of new and positive data on results from a Phase 2 clinical trial evaluating the company’s product Trimesta™ as a treatment for relapsing-remitting multiple sclerosis (RRMS) in women.
Professor Rhonda Voskuhl, director of the Multiple Sclerosis Program at the University of California, Los Angeles (UCLA) David Geffen School of Medicine and the trial’s principal investigator, published the study in the journal Lancet Neurology under the title, “Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial.”
MS relapses are known to significantly decrease during pregnancy. Based on the fact that estriol is the main estrogen hormone produced during pregnancy, researchers hypothesized that this compound could potentially help reduce the relapse rate in women with RRMS. In fact, a pilot study demonstrated that treatment of RRMS patients with oral estriol could significantly reduce the brain lesions associated with MS and induce a favorable shift in the patient’s immune response.
Trimesta is an investigational drug, an oral estriol developed for the treatment of RRMS and MS-related cognitive dysfunction. The goal of this randomized, double-blind, placebo-controlled Phase 2 clinical trial (NCT00451204) was to assess the safety and efficacy of an anti-inflammatory therapeutic combination of oral trimesta (8 mg capsule daily) together with copaxone (glatiramer acetate injection, 20 mg daily) treatment for two years in women with RRMS.
Researchers found that a trimesta plus copaxone combination could induce a decline in MS relapse rate. In terms of safety, the therapeutic combination was found to be generally well-tolerated.
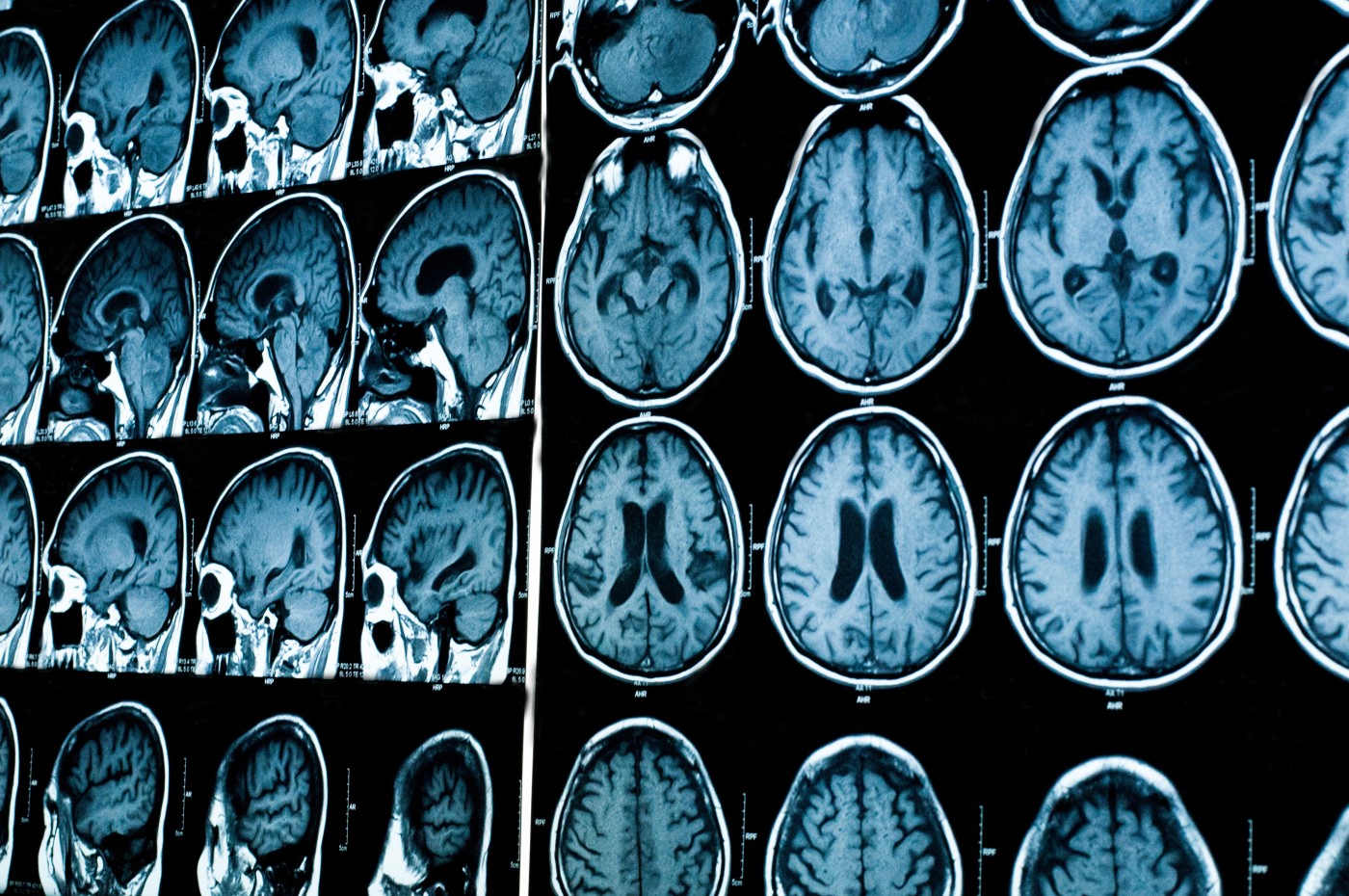
Patients enrolled in the trial (164 in total), among other tests, underwent magnetic resonance imaging (MRI) analyses to evaluate white matter brain lesions and their possible correlation with clinical outcomes. Based on the MRI data, Synthetic Biologics initiated an additional analysis to confirm the results reported by the UCLA researchers. The company is defining the next steps for the development of trimesta together with the neurology community and potential strategic partners, Synthetic Biologics announced in a press release.