Helius showcases PoNS device at CMSC meeting in Nashville
Company highlights progress in accessibility, reimbursement
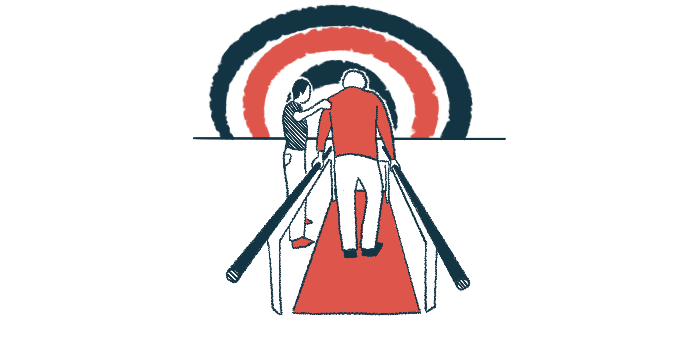
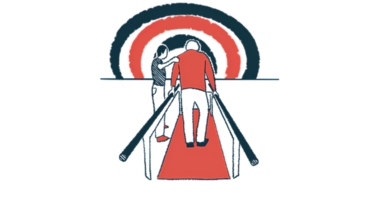
Helius Medical Technologies said it has a booth at a meeting for multiple sclerosis (MS) professionals to showcase its PoNS device and the progress made over the last year in providing access to the neuromodulation device, which is designed to improve walking ability in people with MS.
The device will be exhibited at Booth 500 of the Consortium of Multiple Sclerosis Centers (CMSC) annual meeting May 29–June 1 at the Music City Center in Nashville.
The company said it will demonstrate how its Portable Neuromodulation Stimulator can be used in combination with physical rehabilitation to improve gait in people with MS. It will also highlight recent developments to make PoNS more easily available to patients.
“From the beginning, we have prioritized getting PoNS to as many people with MS and gait deficit as possible,” Dane Andreeff, Helius’ CEO, said in a company press release. “We have been actively meeting and negotiating with insurers and others with the goal of increasing accessibility even before PoNS was commercially available.”
Reimbursement, government partnership, therapist training are key milestones
One major recent development, according to the company, was the assignment of Healthcare Common Procedure Coding System codes covering PoNS by the Centers for Medicare & Medicaid Services, a significant achievement for its reimbursement through Medicare. The final decision on pricing should be effective in October.
Helius has also partnered with Lovell Government Services to make PoNS available through the U.S. Department of Veterans Affairs, the U.S. Department of Defense, and other federal healthcare systems.
And the company said it has trained “a critical mass physical therapists” across the country who design the tailored exercise regimens that are used together with PoNS.
“Each of these achievements represents a win in terms of facilitating access to a technology that makes a meaningful difference,” Andreeff said. “Taken together, they represent important progress in removing barriers that keep people with MS from walking better and doing the things they enjoy most. Removing those barriers and helping more people benefit from PoNS has been our goal since the start.”
PoNS is an approved, noninvasive device consisting of a mouthpiece containing electrodes that’s connected via a cord to a controller. The mouthpiece is placed on the tongue during rehabilitation sessions, delivering mild electrical signals to nerves in the tongue that travel to a brain region involved in motor control.
These signals are designed to promote neuroplasticity, or the brain’s ability to adapt in response to new experiences. When used during movement and coordination tasks in rehabilitation programs, it is expected to strengthen the brain circuits involved in those tasks, increasing the benefits of rehabilitation.
The device is approved in the U.S. as a short-term treatment for walking issues in MS patients with mild to moderate symptoms, in combination with supervised physical therapy, for people ages 22 and older.
The CMSC meeting, which brings together healthcare professionals and researchers involved in MS care, will also feature educational workshops, exhibits, and scientific presentations on advances in MS research findings, clinical studies, and care.