FAQs About the Bioness ‘L300 Go’ for Foot Drop

Earlier this month Bioness announced that the U.S. Food and Drug Administration had cleared its new “L300 Go” functional electronic stimulator (FES). It’s an upgrade of the original “L300” that I’ve been using for more than five years. Without the “L300” strapped to my left leg it’s difficult for me to walk more than 25 or 30 steps, even with two canes.
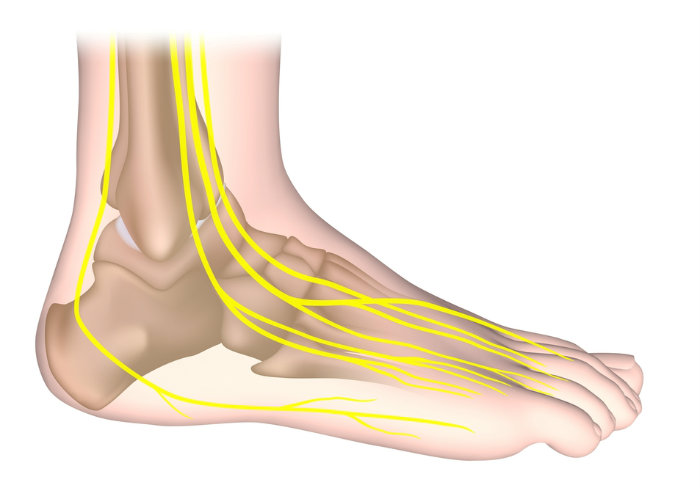
The “L300” sends a low-intensity electrical pulse down a nerve that runs from my knee to my ankle each time I begin tr ying to lift my left leg to walk. That pulse forces my foot to flex upward from my ankle, so my toes don’t drag. (What the docs call “foot drop“). The electrical pulse replaces the signal from my brain to my ankle that’s blocked by my MS.
ying to lift my left leg to walk. That pulse forces my foot to flex upward from my ankle, so my toes don’t drag. (What the docs call “foot drop“). The electrical pulse replaces the signal from my brain to my ankle that’s blocked by my MS.
When Bioness recently announced the FDA clearance of the “L300 Go” a news article was published here on Multiple Sclerosis News Today. That story generated several questions from readers, who wanted a better understanding of the “L300 Go” and how it’s different from the “L300.” So, I’ve been in touch with the folks at Bioness and will try to answer some of the questions I’ve seen posted.
What’s the difference between the “L300” and the “L300 Go?”
As I understand it, the “L300 Go” allows a therapist to use 3-D motion detection system to better adjust an “L300” to make it more responsive to a person’s gait. The 3-D motion detection seems to be the most important new feature. This motion detection system also allows “L300 Go” to be used without the sensor that the “L300” requires to be placed your shoe. That means you’re able to change shoes without having to move a sensor, and even use the device barefoot! (A competitive device, called the “WalkAide,” has had this feature since it came on the market around the same time as the “L300”). The “L300 Go”also responds to motion somewhat faster than the “L300.”
I’ve been using the L300. Does FDA clearance mean that Medicare and insurance will now pay for it?
The FDA clearance was for a new product, the “L300 Go.” The “L300” was cleared by the FDA in the U.S., and received European Commission approval several years ago. The recent clearance doesn’t change the fact that, though Medicare has approved the L300 for use by spinal cord injury patients, Medicare has never approved the “L300” for use by MS patients. As we all know, insurance companies aren’t likely to approve something that Medicare hasn’t approved.
Will Medicare pay for the “L300” for MS patients in the future?
A spokesperson for Bioness tells me that “veterans and their families already have access to our technology as a covered benefit through the Veteran’s Administration. We continue to work with the Centers for Medicare & Medicaid Services (CMS), as well as private/commercial insurance companies, to expand coverage criteria to include more of their beneficiaries.”
Will the price of the “L300” drop now?
I doubt it. I haven’t seen what the price will be for the “L300 Go,” (if that’s even been determined), but Bioness has said it will give a price break to current “L300” users who want to “upgrade.”
Why do you say the FDA “cleared” the “L300 Go,” rather than “approved” it?
It’s a technicality. The FDA “approves” new drugs but it “clears” the use of new medical equipment. Don’t ask me why.
***
[You’re invited to view my personal blog at www.themswire.com.]
Note: Multiple Sclerosis News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The opinions expressed in this column are not those of Multiple Sclerosis News Today, or its parent company, Bionews Services, and are intended to spark discussion about issues pertaining to multiple sclerosis.







Gary Alderson
I have been using the Walkaid since it was released approximately 10-12 years ago. It is a wonderful device thanks to the fact you can wear it without shoes. It is a shame Medicare does not cover it as many of us have nerve damage predominantly in the spinal cord. I believe it is covered for stroke patients.
brad biliter
the Bioness L 300 go isnt covered generally by ins plans for stroke survivors... just thought Id share. But I do own one and it is amazing
Ed Tobias
Thanks for that info, Brad.
Ed
GARY SHAMBLEN
All MS patients getting L-300 need to submit the bill to their insurance for payment. Of course it will be denied. Then with your doctor's help, appeal the denied service including your doctor's letter of medical necessity. If denied again, the next appeal should ask for a Peer to Peer conference between your Dr. and a medical rep. from your insurance co. The company rep. must be of neurological background.
Ed Tobias
Gary,
Thanks for these suggestions. When I purchased my L300 I went through all three steps of the appeal process. However, I didn't know that I could request a peer-to-peer conference between my neuro and that I could request that the insurance company's doctor have a neurological background. So, I was denied. Fortunately, Bioness offered me a "demo" L300 at half price and I shelled out the cash. (I don't know if they still do this). I'm glad that I could afford that expense but I know that there are many who can't. I hope they're able to follow your advice and that they're successful in getting their insurance, or Medicare, to pay for it.
Ed
Ralph
Isn't this alot to ask of my doctor? He is so busy and, I think that it would be difficult for him to spend this much time with my insurance company.
Linda Crooks
Would this device be of any assistance to someone who has no ability left to walk. She's been like that for more than a year now and needs assistance for walking, even a few steps. Could this device do anything of value to her? We are pretty desperate. Until last Easter th is girl could do most things on her own. Now she is almost bedridden. Exercise is a real problem.
Ed Tobias
Hi Linda,
I'm sorry but I suspect she wouldn't be helped. the L300 is designed to correct foot-drop, a foot that drags. It sounds as if the person you're talking about has a disability that is much greater than that. Is multiple sclerosis her diagnosis? What has her doctor recommended? Has a physical therapist evaluated her?
Michele
I have a L300. I need to have maintenance on my current one. I have been unable to get mine working again since I broke my left fibula 3-1-17.
Ed Tobias
Hi Michelle,
Have you contacted the customer service people at Bioness. You can find their contact info at www.bioness.com.
Adriane
You may have injured a nerve distally or in other words at the area of the fracture and thus the device may not be able to stimulate correctly
Dianne Porter
My patient has a L 300 go which is now making a buzzing noise with every step
It was adjusted by a technician 1 week ago and is now not working as well
She is concerned that the device will stop working mid gait and now she is back walking with a stick
Ed Tobias
Hi Dianne,
The L300s can be a little tricky, especially positioning them correctly on the leg if your patient is using the gel electrodes. When I first received my L300 it was fitted by a PT who was trained by Bioness. I also have had some adjustments made and they were done by PTs who are Bioness employees. I've never had a "technician" work on my unit.
I've never heard a buzzing noise, but I know that the hand controller of my old L300 (not the "go") will display error messages which can be decoded in the instruction manual. Has your patient checked that?
I hope she gets the device working. It' certainly been a help to me.
Ed
Dave
I have had the L300 Go knee and thigh cuffs. The clinician programming never stays and when i get home, level 5 which its starts at, shocks the crap out of me. so far ive met with the clinician 5 times with same poor results. It's been almost a year. I need the tablet and app so I can program my own system i paid money for.
Suggestions? Give up on an expensive mistake?
Ed Tobias
Dave,
I wrote another column after this one after test-walking the L300 Go and finding it didn't improve my L300 enough to be worth the expense of the upgrade. However, I was quite disappointed to learn that, before too long, Bioness will stop supporting the original L300. That means no more electrodes and no replacement of the foot sensor or hand control if either stops working.
I emailed Bioness a few weeks ago asking about the current cost of the Go but I received no response.
I've heard this complaint from one other L300 user.
Let me follow up on what you've written and see if I can light a fire under them.
Meanwhile, is anyone else having a problem similar to Dave's? If so, please let me know ASAP.
Ed
Richard Torres
I was diagnosed with primary progressive multiple sclerosis and developed drop foot. After 40+ years of paying into Medicare to be denied products that can help you get back to somewhat of a normal task of walking instead of tripping and falling. Medicare will pay after your fall but will not help you prevent the fall. This is a failure in our Medicare system which will pay exorbitant costs for broken bones but will not prevent them. It's bad enough you're not allowed to work anymore and they make you go broke before granting disability. They deny you the right to preventive health care.
Ed Tobias
You're absolutely right, Richard.
Of course, commercial insurance follows the Medicare lead. When I first used an L300 it was before I was Medicare age. I tried three appeals of my insurance company's denial and I used your argument each time. They just wouldn't buy the argument that they'll wind up saving money by preventing me from falling. Awrggg!!!
Ed
Arlene wallace
My name is Arlene and I have a left drop foot and now I am told I have a drop knee. I am interested in help but have very little income. My Nuerologist only helps people with M.S. I was told there might be grants or some other means of dropping the costs. If not maybe Biomed needs to for the greater good of mankind and not just for the rich who can afford it.
Ed Tobias
Hi Arlene,
I wish I had a good answer for you. The L300 Go is very expensive. So is the Walk Aid,which is a similar unit. It's unfortunate that Bioness has no discount program for those in need. They do have a pay-over-time, option but it doesn't help if you don't have much income to begin with.
There are several foundations that help with the cost of co-pays for medications but I'm not aware of any that will help pay for a mobility aide such as the L300. The Multiple Sclerosis Society of America provides some free mobility aides, but nothing like the L300. Even so, you might want to contact them and see if they have any suggestions: (800) 532-7667 or email [email protected]. Let us know if you have any success.
Good luck,
Ed
Dianne Pernak
Has there been any movement or update status of medicare approving the L300 for MS patients?
Recently been denied by BSBC indicating that the NessL300 is considered experimental.
Dianne
Ed Tobias
Hi Dianne,
Unfortunately, no. Medicare still won't pay for it and I doubt that will change anytime soon. I finally broke down and upgraded to the L300 Go. I could only do that because Bioness has a deal with Care Credit to pay over time with no interest.
Here's a column I recently wrote about my experience wit the L300 Go that might interest you. http://multiplesclerosisnewstoday.com/columns/2019/12/03/a-bioness-l300-go-is-now-on-my-leg/
Good luck,
Ed