FAQs about stem cell therapy for MS
There is no cure for multiple sclerosis (MS), but studies suggest that some stem cell therapies can slow the progression of the disease. In particular, autologous hematopoietic stem cell transplant is seen as generally effective for reducing disease activity and slowing disability progression, notably in younger individuals with aggressive relapsing disease that is resistant to highly effective disease-modifying medications. The benefits of mesenchymal stem cells are less clear, though early clinical trials also suggest improvements in clinical outcomes.
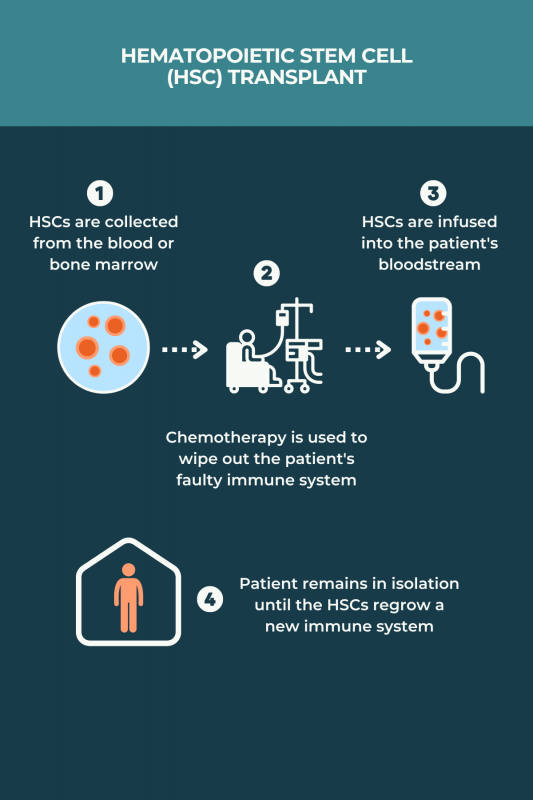
Due to the risks associated with autologous hematopoietic stem cell transplant (aHSCT), more commonly known as stem cell therapy, the procedure should only be performed at a specialist center accredited by the Foundation for Accreditation of Cellular Therapy, known as FACT. People with multiple sclerosis interested in this treatment are advised to talk with their care team about whether they are good candidates for aHSCT, and to find centers that provide this type of care.
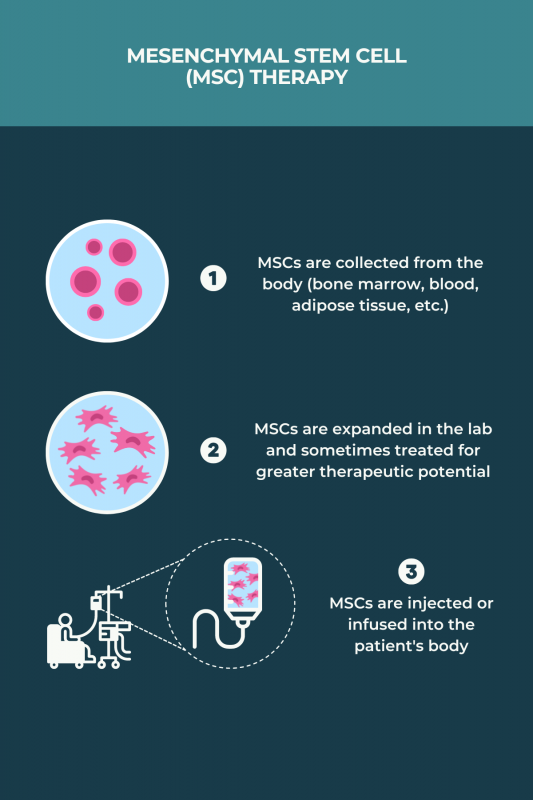
According to a large analysis of published studies, about two-thirds of people with multiple sclerosis (MS) who underwent an hematopoietic stem cell transplant did not have any disease activity within five years after the procedure. Trials of mesenchymal stem cells (MSC) conducted to date were mostly small and without a control group, so the efficacy of MSC-based treatments in MS remains unclear.
Hematopoietic stem cell transplants, or blood stem cell transplants, are generally more effective in individuals with relapsing-remitting multiple sclerosis than in those with progressive forms of the disease. Response to this treatment also tends to be better in patients who are younger than 50 and early on in the disease course. Ongoing trials are testing mesenchymal stem cell therapies across all multiple sclerosis types, but it’s not yet known if any disease type benefits the most from these therapies.
No form of stem cell therapy is specifically approved by the U.S. Food and Drug Administration to treat multiple sclerosis (MS). Nonetheless, the individual medications and procedures used in hematopoietic stem cell transplants have been approved by the regulatory agency, meaning that MS patients can access the treatment in the U.S. Stem cell transplant also is supported by the National MS Society to treat relapsing MS patients with highly active disease.
Related Articles

 Fact-checked by
Fact-checked by