FSD Asking to Open Trial of Lucid-MS, Myelin-protecting Therapy
Application to Health Canada for Phase 1 safety and tolerability study
Written by |
FSD Pharma has submitted an application to Health Canada asking to start a Phase 1 clinical trial of Lucid-MS, the company’s experimental and myelin-protective oral therapy for people with multiple sclerosis (MS).
The trial will not involve patients and is designed to investigate the safety and tolerability of the compound, which also is intended to treat all forms of MS. The company did not disclose additional details of the proposed study.
A similar request is planned for the U.S. Food and Drug Administration after the application’s appraisal by Canadian regulators.
“We are optimistic about the potential of Lucid-MS and we are eager to initiate this clinical trial taking this one step closer to the patients,” Lakshmi Kotra, PhD, CEO of Lucid Psycheceuticals, a wholly owned subsidiary of FSD Pharma, said in a FSD press release.
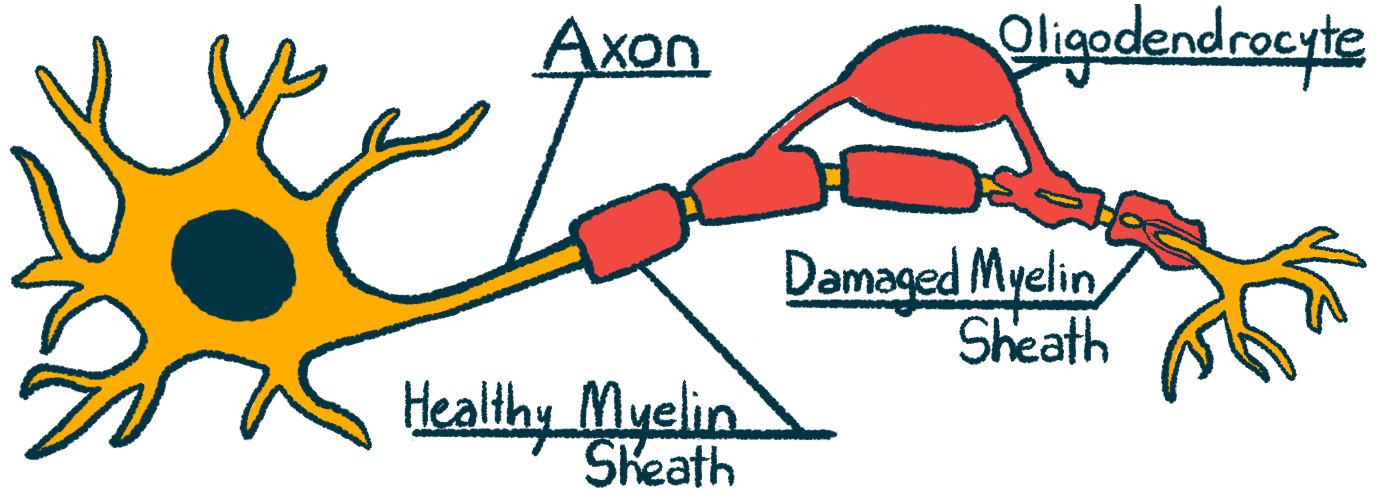
Treatment prevented damage to myelin sheath in MS mouse model
MS is caused by inflammation in the brain and spinal cord, which damages the myelin sheath, a fatty wrapping around nerve fibers that helps them send electric signals. This damage disrupts neurological signaling, ultimately giving rise to MS symptoms.
All currently available treatments for MS are immunomodulatory — that is, they work by reducing the activity of the immune system to lessen disease-driving inflammation.
There are over a dozen approved treatments for relapsing types of MS, where symptom worsening is driven mainly by new inflammatory damage. Far fewer treatments are available for progressive forms of MS, which are driven mainly by the gradual accumulation of nerve damage over time.
No therapies are available that can help to restore damaged myelin.
“Multiple Sclerosis is a debilitating disease without any cure, relegating patients and caregivers to significantly lower quality of life. There is an intense effort for disease-modifying treatments to address unmet needs especially for the treatment of progressive MS, and potentially that are non-immunomodulatory,” Kotra said.
Lucid-MS is a patented neuroprotective therapy that has been shown to help prevent myelin damage in disease models, according to FSD.
In mice with experimental autoimmune encephalomyelitis, which is commonly used to model MS, treatment with the experimental therapy substantially lessened disease severity. In particular, the treatment helped mice with hindlimb and tail paralysis regain limb mobility, and animals recovered completely after two months on treatment.
“Lucid-MS is a promising first-in-class agent with a novel mechanism of action,” Kotra said, adding that the treatment “is non-immunomodulatory in its mechanisms of action based on current evidence” and has the “potential to address progressive stages of the disease.”



