Disease-modifying therapies for multiple sclerosis
In multiple sclerosis (MS), the body’s immune system launches an inflammatory attack in the central nervous system that damages the myelin sheath, a covering around nerve cells that is important for their function. The resulting nerve damage gives rise to the disease’s symptoms.
Disease-modifying therapies, or DMTs, are a type of treatment that can alter the course of MS — by reducing the risk of relapses, decreasing disease activity as assessed on MRI scans, and/or slowing the accumulation of MS symptoms that interfere with daily life.
All of the DMTs now available to treat MS essentially work by lowering the activity of the immune system, thereby reducing the severity of the inflammatory attack that drives the disorder.
Notably, although disease-modifying therapies can help prevent MS from getting worse, they do not reverse damage in the nervous system that has already occurred. Thus, they don’t usually improve symptoms in day-to-day life. As such, it is generally recommended that MS patients start treatment with a DMT as early as possible.
DMT approval by MS type
When regulatory authorities approve a DMT, the approval will specify which types of MS the medication is intended to treat. MS is generally categorized into four types:
- clinically isolated syndrome (CIS)
- relapsing-remitting MS (RRMS)
- secondary progressive MS (SPMS)
- primary progressive MS (PPMS)
MS also is categorized based on whether or not the patient experiences relapses, or bouts of sudden worsening of disease symptoms.
Active inflammation generally plays a central role in the progression of relapsing disease, and many inflammation-lowering medications have proven effective. In the U.S., more than 20 DMTs are approved to treat relapsing forms of MS, which generally include CIS, RRMS, and active SPMS. In other countries, some therapies approved for all these forms in the U.S. are only authorized for RRMS.
While relapsing MS is primarily due to active inflammation, non-relapsing forms of the disease — including PPMS and non-active SPMS — are driven mainly by the gradual death and dysfunction of nerve cells (neurodegeneration). These progressive MS types have historically proven to be more difficult to treat. In the U.S., the only approved DMT for PPMS is Ocrevus, and the sole approved therapy for non-active SPMS is mitoxantrone.
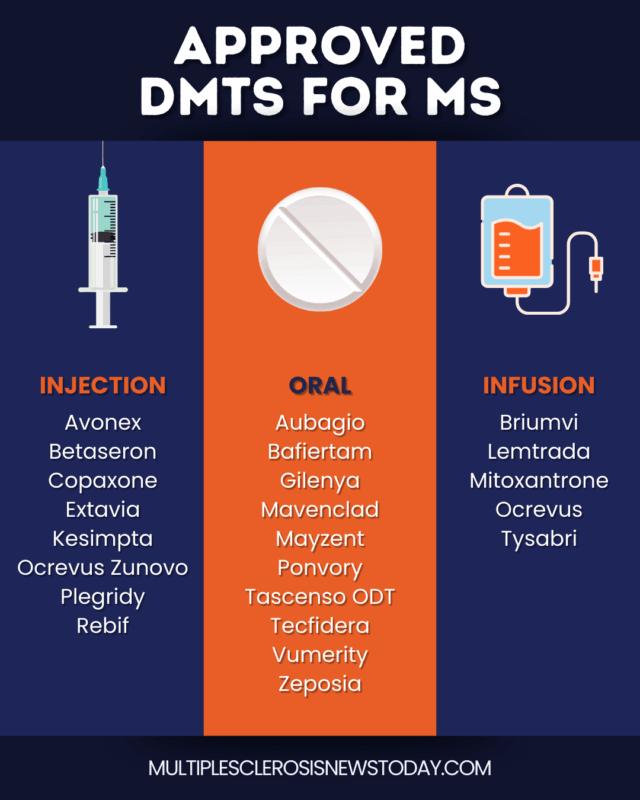
Approved DMTs for MS in the US
DMTs administered by injection
- Avonex (interferon beta-1a): approved for relapsing forms of MS, administered via injection into the muscle once per week.
- Betaseron (interferon beta-1b): approved for relapsing types of MS, and given by injection under the skin every other day. Extavia is a generic form of Betaseron.
- Copaxone (glatiramer acetate): approved to treat relapsing MS types, administered via under-the-skin injections either once per day or three times per week. Generic forms of this medication are available.
- Kesimpta (ofatumumab): an antibody-based therapy approved for relapsing forms of MS, given by monthly injections under the skin.
- Plegridy (peginterferon beta-1a): an approved treatment for relapsing MS types, given every two weeks; it can be administered via either injection into the muscle or under the skin.
- Rebif (interferon beta-1a): approved to treat relapsing MS types, given via injection under the skin three times per week.
Oral DMTs
- Aubagio (teriflunomide): a once-daily tablet approved for relapsing forms of MS. Generic forms of this medication are available.
- Bafiertam (monomethyl fumarate): a bioequivalent of Tecfidera for treating relapsing forms of MS; taken twice per day.
- Gilenya (fingolimod): a therapy approved for relapsing MS types and taken once per day.
- Mavenclad (cladribine): a short-course oral treatment for relapsing MS, given in four cycles, each lasting about two weeks, over the course of two years. Due to its safety profile, Mavenclad usually is recommended for patients who have not responded to other DMTs.
- Mayzent (siponimod): a once-daily therapy approved for relapsing MS types.
- Ponvory (ponesimod): a therapy approved for relapsing MS forms and taken once per day.
- Tecfidera (dimethyl fumarate): taken twice per day; approved to treat relapsing forms of MS. Generic forms of Tecfidera are available.
- Vumerity (diroximel fumarate): an approved treatment for relapsing MS, taken twice per day. Vumerity is designed to deliver the same active ingredient as Tecfidera, but with fewer digestive side effects.
- Zeposia (ozanimod): a once-per-day therapy approved for relapsing forms of MS.
DMTs administered by infusion
- Briumvi (ublituximab): an antibody-based medication approved for relapsing forms of MS, given in hour-long infusions every six months.
- Lemtrada (alemtuzumab): approved antibody-based therapy for relapsing forms of MS, given in two rounds of four-hour infusions across several days, one year apart. Because Lemtrada can cause severe complications, it is generally only recommended for people who have not responded to at least two other MS treatments, and is only available in the U.S. through a restricted distribution program.
- Mitoxantrone: the only therapy approved for all forms of SPMS, also authorized for RRMS patients who have significant symptoms between relapses. It is given via infusion every three months.
- Ocrevus (ocrelizumab): an antibody-based therapy approved for relapsing forms of MS, and the only treatment approved for PPMS. This therapy is given via infusions every six months.
- Tysabri (natalizumab): approved antibody-based therapy to treat relapsing MS types, given via hour-long infusions every four weeks. Tysabri is only available in the U.S. through a restricted distribution program because it may increase the risk of serious infections.
Strategies for selecting a DMT
There are several factors that go into the selection of which DMT to use. Generally, when healthcare professionals treat a person with MS for the first time, they will use one of two main strategies: escalation or induction.
In the escalation strategy, which historically has been more common, patients are first treated with a moderately effective DMT that has a well-established safety profile. This is called “first-line” therapy — that is, a primary treatment usually recommended for a certain disease. Then, if the person does not respond adequately to treatment, or is unable to tolerate its side effects, that patient may be switched to a more potent therapy. These are called “second-line” treatments. But they may, however, carry a higher risk of side effects.
DMTs that are commonly used as first-line treatments in the escalation strategy include:
- fumarates (Bafiertam, Tecfidera, Vumerity, and generic forms)
- glatiramer acetate (Copaxone and generics)
- interferon therapies, such as Avonex, Betaseron, Extavia, Plegridy, and Rebif
- teriflunomide (Aubagio and generics)
The induction strategy, on the other hand, involves the use of highly effective DMTs — usually second-line therapies — right from the start. This is often seen as a more aggressive approach to treatment and is usually used in people with very active disease. High-efficacy DMTs are usually more powerful in reducing disease activity, but also tend to be associated with greater risks and side effects. Thus, their safety profile might not be an acceptable therapeutic strategy for all MS patients.
DMTs that may be used in induction treatment include:
- antibody-based therapies, like Ocrevus and Kesimpta
- Gilenya, Mayzent, and Zeposia (all these therapies target the same receptor, called S1P, in certain immune cells)
- Lemtrada
- Mavenclad
- mitoxantrone
- Tysabri
Multiple Sclerosis News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Related articles

 Fact-checked by
Fact-checked by