Primary progressive multiple sclerosis (PPMS)
Approximately 10% to 15% of people who develop multiple sclerosis (MS) are diagnosed with primary progressive multiple sclerosis, or PPMS. In this form of the disease, MS symptoms steadily worsen over time, starting from disease onset.
MS is a chronic neurological disorder caused by inflammation that damages nerve fibers in the central nervous system, which includes the brain, spinal cord, and optic nerves. MS is classified into different types based on how disease symptoms manifest and evolve.
What is primary progressive MS?
Most people with MS don’t begin experiencing steady symptom worsening immediately. Instead, they’ll be diagnosed with relapsing-remitting MS (RRMS), where periods of sudden symptom worsening — called relapses, flares, or exacerbations — are interspersed with periods of remission when symptoms ease and remain relatively stable over time.
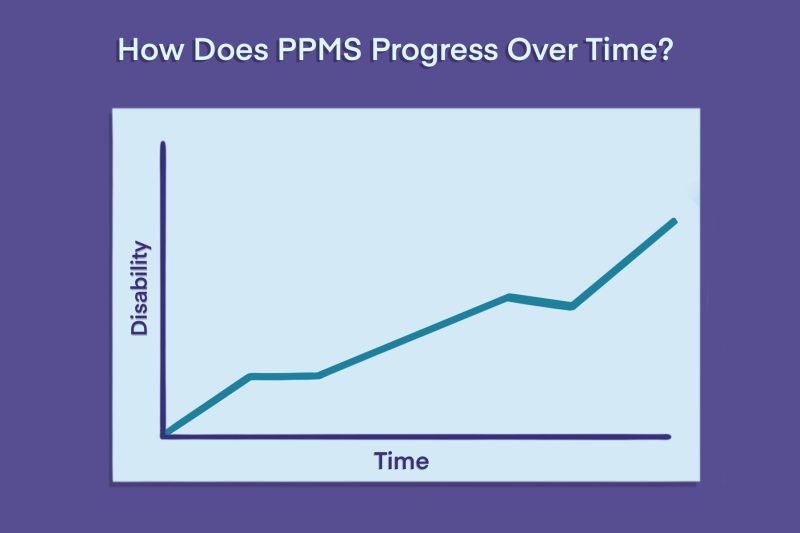
By contrast, beginning at disease onset, people with PPMS experience a gradual and steady worsening of symptoms without periods of recovery. MS disability progression occurs even in the absence of relapses, although up to around 30% of patients may experience occasional flares.
People with RRMS may eventually transition to a progressive form of the disease called secondary progressive MS (SPMS) that has similar characteristics to PPMS.
| Disease type | Features |
|---|---|
| Primary progressive MS | Symptoms gradually worsen beginning at disease onset, independent of relapse activity |
| Relapsing-remitting MS | Periods of rapid symptom worsening (relapses) are interspersed with periods of relative stability and symptom easing (remission) |
| Secondary progressive MS | A progressive stage of disease that follows RRMS; symptoms begin to gradually worsen, independent of relapse activity |
There are a couple of other ways that physicians may also classify PPMS. They are:
- active or nonactive, based on whether the patient experiences occasional relapses or has evidence of new disease activity on MRI scans.
- with or without progression, based on whether disability is progressing or staying stable over time.
Like other forms of MS, PPMS is an autoimmune disease caused by mistaken immune attacks on cells in the nervous system. While bouts of inflammation from these attacks can explain the symptom flares of relapsing disease, people with PPMS tend to experience less active inflammation. The progression of PPMS symptoms instead is mainly driven by nerve cell damage and loss.
PPMS patients also tend to have fewer areas of damage, or lesions, in the brain, but more spinal cord lesions, relative to other MS types.
Although relapsing MS is substantially more common in women than men, PPMS affects the sexes at roughly equal rates. PPMS also usually develops, on average, about a decade later in life than relapsing MS. Most people with PPMS are diagnosed in their 40s and 50s, though earlier or later diagnoses can also occur.
Symptoms of PPMS
People living with PPMS experience many of the same MS symptoms as those with relapsing forms of the disease.
However, weakness in leg muscles and difficulty walking are typically more common and persistent in PPMS. For most people with PPMS, trouble walking is the first obvious symptom. Other common PPMS symptoms include:
- fatigue
- numbness or other unusual sensations, referred to as dysesthesias
- vision problems
- spasticity, or abnormally tight or stiff muscles
- difficulty controlling the bladder and bowels
- sexual dysfunction
- cognitive and emotional challenges
The rate of disease progression in MS varies widely from person to person, but in general, symptoms accumulate more quickly in PPMS than in relapsing disease types.
Diagnosis of PPMS
In early versions of the McDonald criteria — the clinical guidelines used to make an MS diagnosis — progressive and relapsing forms of MS were diagnosed using separate diagnostic pathways. However, the most recent revision, in 2024, introduced a unified framework for diagnosing all types of MS.
The process typically involves clinical testing to identify signs of inflammation and nerve damage consistent with MS, as well as to rule out other possible diagnoses. This includes:
- a thorough physical exam and medical history to track symptoms
- MRI scans to identify lesions
- analyses of cerebrospinal fluid (CSF), the liquid that surrounds the brain and spinal cord, to identify signs of MS-related inflammation
Different combinations of evidence can confirm an MS diagnosis. For example, lesions in several parts of the brain and spinal cord may be sufficient to establish a diagnosis, but if the damage is more limited, cerebrospinal fluid findings may also be required.
The 2024 McDonald criteria still include two distinctions specific to PPMS: They are that:
- patients must experience at least one year of continual disease progression — that is, steadily worsening symptoms without periods of remission
- the presence of at least two lesions in the spinal cord can substitute for evidence of more widespread damage in the brain
A PPMS diagnosis can take a long time, especially in cases when neurologic symptoms have just begun. Diagnostic delays are usually longer in PPMS than in relapsing forms of MS.
Treatment of PPMS
Treatment options are more limited for PPMS than for relapsing forms of the disease. To date, there is only one disease-modifying therapy (DMT) approved in the U.S. to treat PPMS: Ocrevus (ocrelizumab).
The therapy, which earned its U.S. approval in 2017, was shown in clinical trials to slow disability progression in people with PPMS, especially when started early in the disease course and used continuously.
The original formulation is given via infusions into the bloodstream and is widely approved for PPMS globally. A version that’s injected under the skin, called Ocrevus Zunovo (ocrelizumab and hyaluronidase-ocsq), is also now available in some regions, including the U.S. and Europe.
Other experimental therapies are in development, and several clinical trials involving participants with PPMS are ongoing.
While Ocrevus is the only medication that can modify the disease course, PPMS treatment may also involve medications to manage symptoms and improve quality of life, including:
- spasticity: botulinum toxin products (such as Dysport and Botox), muscle relaxants, and certain anti-anxiety medications
- fatigue: none specifically approved for MS fatigue, but lifestyle changes and off-label medications may help
- walking issues: Ampyra (dalfampridine), Portable Neuromodulation Stimulator (PoNS) device
- bladder dysfunction: Botox or other medications to relax the bladder muscles
- constipation: over-the-counter stool softeners, laxatives, enemas, or suppositories
- erectile dysfunction: medications to increase penile blood flow
- nerve pain: none specifically approved for MS, but various anti-seizure or antidepressant medications can be used off-label
- mental health or mood problems: antidepressants and anxiety medications; Nuedexta (dextromethorphan and quinidine) for sudden bursts of uncontrollable laughter or crying, called the pseudobulbar affect
Outlook of PPMS
Because specific symptoms and rates of progression vary widely in MS, the prognosis of PPMS differs substantially between individuals. People with PPMS generally experience a faster accumulation of disabling symptoms — and, consequently, more pronounced difficulties in daily life — compared with those with relapsing disease forms. However, no form of MS is inherently fatal, and the availability of DMTs has improved MS prognosis substantially.
After a PPMS diagnosis, patients should talk to their doctors and other healthcare professionals to establish a treatment and care plan. In addition to medications, complementary care approaches can help make living with PPMS easier. This could include:
- lifestyle adjustments, such as eating a healthy diet, practicing appropriate exercise, and quitting smoking
- physical therapy, to minimize muscle weakness, restore movement, and ease symptoms like fatigue
- occupational therapy, to make daily activities easier and prolong functional independence
- talk therapy, counseling, or support groups for mental health
Multiple Sclerosis News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by 

